Tech Note — The Difference Between VFA and FT-IR Spectra
The basic characteristics of variable filter array (VFA) spectrometers, both near- and mid-infrared, make them well suited for routine infrared analysis applications outside and inside the laboratory. The performance of this spectrometer is discussed here.
The basic characteristics of variable filter array (VFA) spectrometers, both near- and mid-infrared, available from several manufacturers, make them well suited for routine infrared analysis applications outside and inside the laboratory. Although the infrared data gathered by a VFA spectrometer is similar to that obtained from an FT-IR instrument, it differs in one key respect — resolution. In the mid-IR region, FT-IR spectra usually are recorded at resolutions between 0.5 cm-1 and 8 cm-1 . A VFA spectrometer with 64 pixels has a theoretical resolution of 16 cm-1 . Because of crosstalk from pixel to pixel, both thermal and optical, the resolution is reduced further to the neighborhood of 32 cm-1 .
What effect does low resolution have upon the performance of a VFA spectrometer? To answer this question, we have used a VFA spectrometer in several different applications to determine the effect, if any, of low resolution.
Raw material verification. A company uses a solvent that comes in three isomers, each specific to a different process. Sending a sample to the laboratory can take over an hour for verification, delaying the offloading of the material. Figure 1 shows VFA spectra of the three isomers. Even though the spectral information in the VFA spectra is considerably less than that of FT-IR traces, there is enough data from the VFA spectrometer to verify the isomer in the shipment. Because a VFA spectrometer has no moving parts and no free air path, it is unaffected by atmospheric absorptions and can be situated at the receiving dock. In less than 5 min, the incoming raw material can be verified and off-loaded to the correct location.
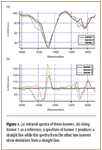
Figure 1. (a) Infrared spectra of three isomers. (b) Using Isomer 1 as a reference, a spectrum of Isomer 1 produces a straight line while the spectra from the other two isomers show deviations from a straight line.
Process monitoring. In a process, a material that has an absorption band "A" between 8.5 and 9 µm (Figure 2) converts to a material that has band "B" at 7.0 µm. The reaction can take several hours. The figure shows spectra periodically taken by the VFA instrument situated close to the production line, which follow the switch from "A" to "B". Again, in this situation, the resolution is sufficient to tell when a reaction is complete.
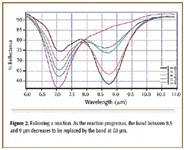
Figure 2. Following a reaction. As the reaction progresses, the band between 8.5 and 9 µm decreases to be replaced by the band at 7.0 µm.
Flexible film manufacturing. There are many areas in flexible film manufacturing in which an onsite test is invaluable. With multilayer films, rolls of film are laminated together with individual rolls, often having different top and bottom materials. Figure 3 shows spectra of common materials used in flexible film production. The spectra differ enough so that an operator in the production area can verify the film and orient it in the right direction for lamination. In the warehouse, rolls of finished films can be verified on-site before shipment to ensure that the customer is receiving the desired product. Another application involves measuring an inside layer thickness, such as nylon, in a multilayer film. Nylon has an absorbance different from the spectra of the outside polyethylene layers, which can be distinguished with a lower resolution VFA spectrometer.
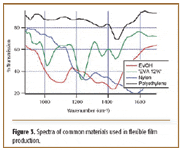
Figure 3. Spectra of common materials used in flexible film production.
FT-IR Spectra
"White powder" verification. Partly as a result of 9/11, and partly because materials are being examined frequently for identification purposes, mid-IR FT-IR spectrometers have become widely used for this purpose to the extent that libraries containing thousands of spectra of known materials have been constructed. Is it feasible to use a VFA spectrometer to search the libraries for identification purposes?
The spectra of common pharmaceutical products shown in Figure 4 are a good example of the differences between VFA spectra and FT-IR spectra. While each VFA spectrum is distinctly different from the others, each also is quite different from its FT-IR counterpart. Because of these substantial differences, it appears to be impractical to compare spectra from FT-IR libraries directly.
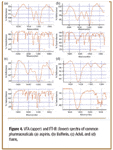
Figure 4. VFA (upper) and FT-IR (lower) spectra of common pharmaceuticals (a) aspirin, (b) Bufferin, (c) Advil, and (d) Tums.
On the other hand, if one examines the VFA spectra, it becomes apparent that each product has a characteristic VFA spectrum, sometimes more readily identifiable than its FT-IR spectrum, so libraries of VFA spectra could serve to identify various groups of materials. A pharmaceutical company might use one or two dozen materials routinely; it would be easy to build VFA spectral libraries with the compounds. Likewise, a law enforcement agency might be concerned with a fixed number of narcotics or poisons for which libraries could be created.
While it does not appear that the VFA spectrometer will replace the FT-IR instrument as a general purpose unknown material identifier, it can be used for this purpose when a relatively small number of materials is involved. Because VFA spectrometers are small, rugged, and usually contain no moving parts or exposed optical paths, they can be practical alternatives to FT-IR instruments when applicable.
It is possible to degrade FT-IR spectra from spectral libraries to lower resolution, as in Figure 5, so that they tend to match VFA spectra. However, in so doing, search programs might be less specific and could produce a number of possible hits rather than one or two.
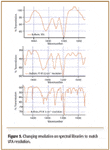
Figure 5. Changing resolution on spectral libraries to match VFA resolution.
Low Resolution Effects Can Be Positive
From the examples presented here, it is apparent that low-resolution mid-IR data is not necessarily a deterrent and often can have a positive effect. As the examples show, low-resolution spectra frequently contain gross differences between spectra of materials, so that visual verification of materials is feasible. Automatic identification from a VFA spectra library also is feasible. Although FT-IR spectra can contain much more detail, visual differences tend to be less apparent, and therefore, require a more detailed systematic evaluation for positive identification. Obviously, both high-resolution FT-IR spectra and low-resolution VFA spectra are useful, depending upon the application.
Note: The VFA spectra shown in this article were run on a Wilks InfraSpec VFA spectrometer. The FT-IR spectra were run on a Nicolet Model 5PCFTIR spectrometer.
Paul Wilks is president of Wilks Enterprise, Inc. (South Norwalk, CT).

High-Speed Laser MS for Precise, Prep-Free Environmental Particle Tracking
April 21st 2025Scientists at Oak Ridge National Laboratory have demonstrated that a fast, laser-based mass spectrometry method—LA-ICP-TOF-MS—can accurately detect and identify airborne environmental particles, including toxic metal particles like ruthenium, without the need for complex sample preparation. The work offers a breakthrough in rapid, high-resolution analysis of environmental pollutants.
The Fundamental Role of Advanced Hyphenated Techniques in Lithium-Ion Battery Research
December 4th 2024Spectroscopy spoke with Uwe Karst, a full professor at the University of Münster in the Institute of Inorganic and Analytical Chemistry, to discuss his research on hyphenated analytical techniques in battery research.
Mass Spectrometry for Forensic Analysis: An Interview with Glen Jackson
November 27th 2024As part of “The Future of Forensic Analysis” content series, Spectroscopy sat down with Glen P. Jackson of West Virginia University to talk about the historical development of mass spectrometry in forensic analysis.
Detecting Cancer Biomarkers in Canines: An Interview with Landulfo Silveira Jr.
November 5th 2024Spectroscopy sat down with Landulfo Silveira Jr. of Universidade Anhembi Morumbi-UAM and Center for Innovation, Technology and Education-CITÉ (São Paulo, Brazil) to talk about his team’s latest research using Raman spectroscopy to detect biomarkers of cancer in canine sera.