Using ICP-OES to Improve Lithium-Ion Battery Performance and Reduce Waste
As the demand grows for lithium-ion (Li-ion) batteries, their performance requirements and environmental impact increase. Battery performance strongly depends on the composition of the cathode materials, requiring precise elemental ratios. Meanwhile, disposing spent batteries can have a negative environmental impact, which can be greatly reduced through recycling. Inductively coupled plasma–optical emission spectroscopy (ICP-OES) provides solutions in both of these areas. By using high-precision ICP-OES, precise measurements can be made to accurately determine compositions of a variety of different cathode materials. In battery recycling, ICP-OES meets the requirements of being a multielement technique with a wide dynamic range and the ability to handle complex matrices. Therefore, it can measure both high-concentration and impurity elements resulting from the incineration of spent batteries, providing recycling facilities information about the elements present and their levels so that recoveries can be optimized.
The performance of lithium-ion (Li-ion) batteries is strongly influenced by the composition of the cathode materials: different materials have different performance characteristics. Examples of cathodes include LiCoO2, LiMn2O4, LiNixMnyCozO2, LiFePO4, LiAlFePO4, and LiMnFePO4, among others, with new ones continuously being developed. However, for a given material, the exact composition impacts battery performance. For example, in LiNixMnyCozO2 (NMC), the molar ratios of Ni:Mn:Co affect battery performance, and they can be adjusted depending on the battery’s intended use (1). Therefore, it is critical to determine the molar ratios of cathode materials elements accurately, precisely, and consistently.
One of the issues facing the Li-ion battery industry is finding enough lithium and other cathode elements to meet the demand (2). Larger quantities can be extracted from the earth, but this process comes with a negative environmental impact (3). To address these challenges, battery recycling is becoming more prominent so that important elements can be reused. Two common methods of battery recycling are pyrometallurgy and hydrometallurgy, both of which produce a complex mixture of carbon, ash, graphite, residual electrolyte, cathode elements, and impurity elements known as “black mass.” To determine the quantities of usable elements that can be extracted, the black mass must be analyzed for cathode material elements and elemental impurities.
Inductively coupled plasma–optical emission spectroscopy (ICP-OES) can help in both of these scenarios. With its multi-element capability, ability to handle complex matrices, and wide dynamic range, ICP-OES is uniquely suited to address the analytical requirements of battery production and recycling. Because of its robust plasma and ability to read multiple wavelengths simultaneously, ICP-OES provides the consistent, high-precision elemental ratios required to characterize cathode materials during battery production. The wide dynamic range allows both cathode and impurity elements to be measured during battery recycling, providing important information so that recoveries can be optimized.
This work shows how ICP-OES can assist in both cathode production and battery recycling.
Experimental
Sample Preparation
A 0.1 g quantity of nickel manganese cobalt (NMC) or LiFePO was combined with 3 mL hydrochloric acid (HCl; concentrated) in sample digestion tubes, which were then loosely capped and heated at 120 °C for 30 min in a sample preparation block. After the solutions cooled to room temperature, they were diluted to 50 mL with deionized water and then filtered to remove any graphite. For analysis, the filtered samples were diluted another 10 times with deionized water.
Because of the presence of carbon, ash, and graphite in the black mass sample, it is extremely difficult to digest. Instead, metals were leached from black mass by placing 50 mg of sample in a sample digestion tube, followed by 5 mL of aqua regia. The tubes were loosely capped and heated at 120 °C for 30 min. After the tubes cooled to room temperature, they were diluted to 50 mL with deionized water for analysis.
Analysis
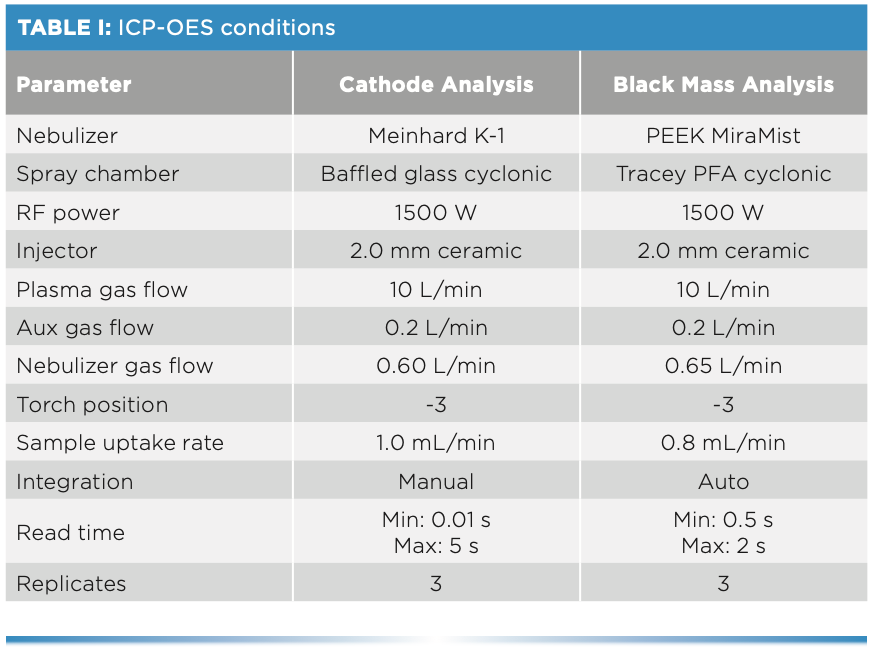
All analyses were done on a PerkinElmer Avio 550 Max fully simultaneous ICP-OES instrument using the conditions and wavelengths listed in Tables I and II. For the cathode materials, a technique known as high-precision ICP-OES was used (4), which involves using manual integration to measure the internal standard and analyte at exactly the same time by setting their read and integration times to be equal. In this manner, the internal standard compensates in real time for any variation in analyte signal, which can be because of plasma flicker or pulsations from the peristaltic pump. The result is excellent precision, with relative standard deviation (RSD) values of 0.2% or less.
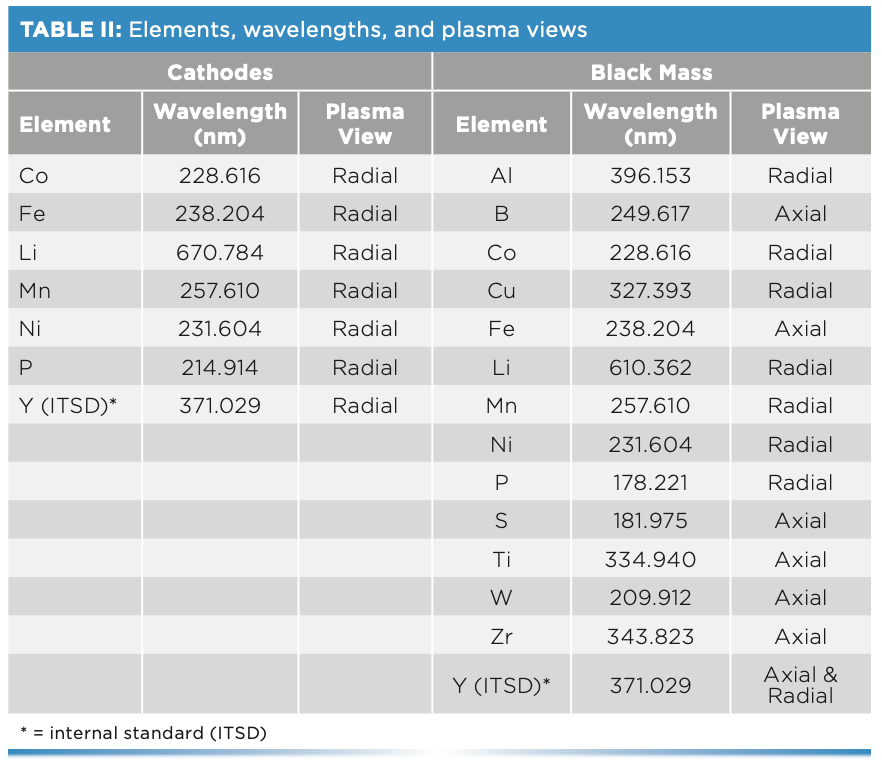
The black mass sample was first analyzed with SmartQuant, a semi-quantitative technique within Syngistix for ICP software, which gives a quick overview of the elemental composition of a sample. Once the major and impurity components present were determined, a comprehensive quantitative analysis was performed on those elements to attain more accurate results.
An important difference between the analyses was the sample introduction systems. A standard nebulizer and spray chamber were used for the cathode analysis, but because black mass can contain hydrofluoric acid (HF), an HF-re- sistant sample introduction system was used.
Results and Discussion
Li-ion Battery Production
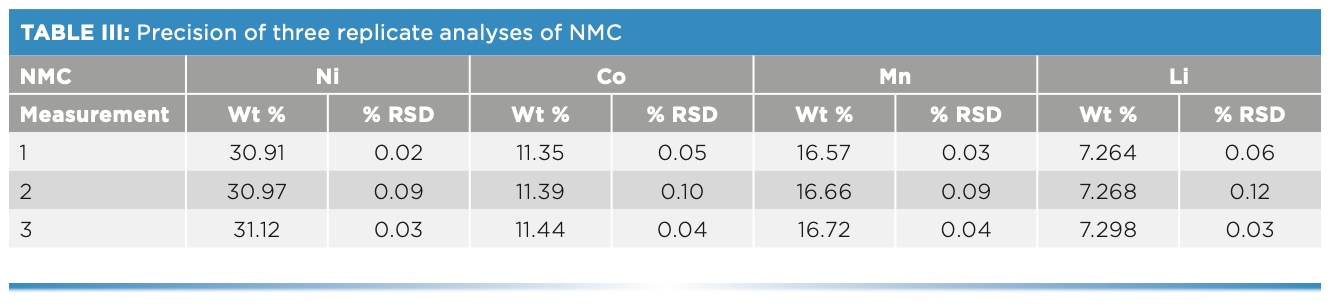
Because the composition of cathode materials is critical to the performance of Li-ion batteries, the elemental ratios of cathode materials must be determined with high precision, both among replicates within a sample and over time as multiple samples are analyzed. To demonstrate high precision between replicates, an NMC sample was measured consecutively three times. As shown in Table III, both the weight percent and precision (RSD) for all elements were <0.1%, demonstrating the precision attainable.
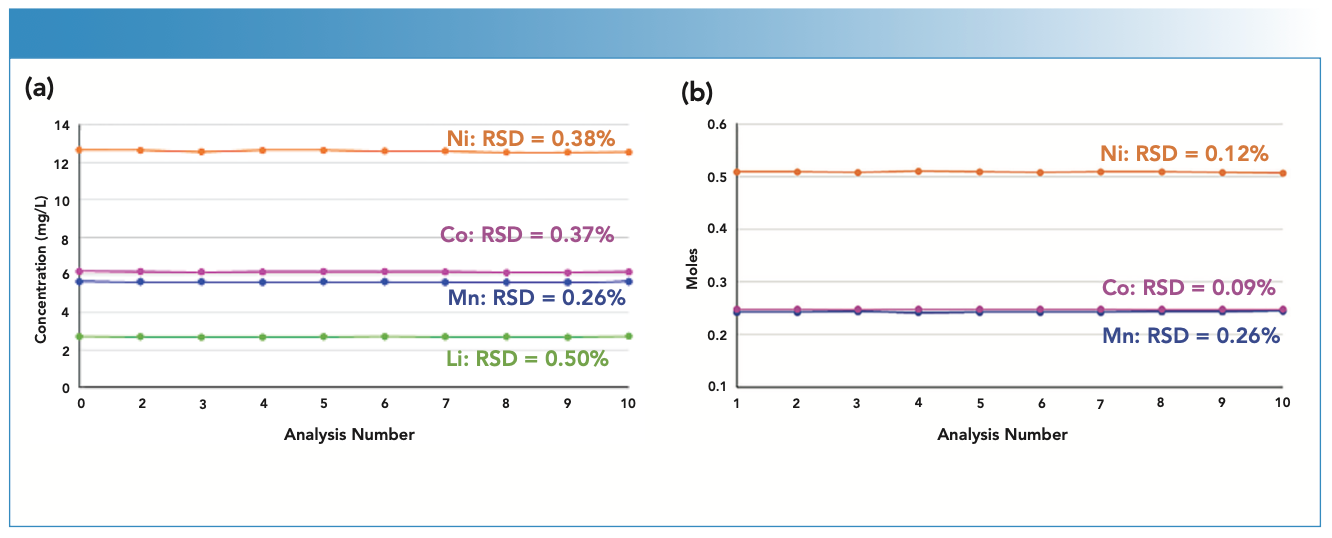
To assess stability, the NMC sample was measured 10 times over 20 min to determine the stability of the measurements. As shown in Figure 1, both the RSDs of the concentrations in solution and the molar ratio are very consistent, with concentration RSDs less than 0.5% and molar ratios less than 0.3%. Taken together, these results demonstrate the ability of ICP-OES to provide the high precision and consistency required for lithium-ion battery production.
FIGURE 1: (a) Concentration and (b) molar ratio stability of Ni, Mn, Co, and Li in an NMC sample from 10 measurements over 20 min.
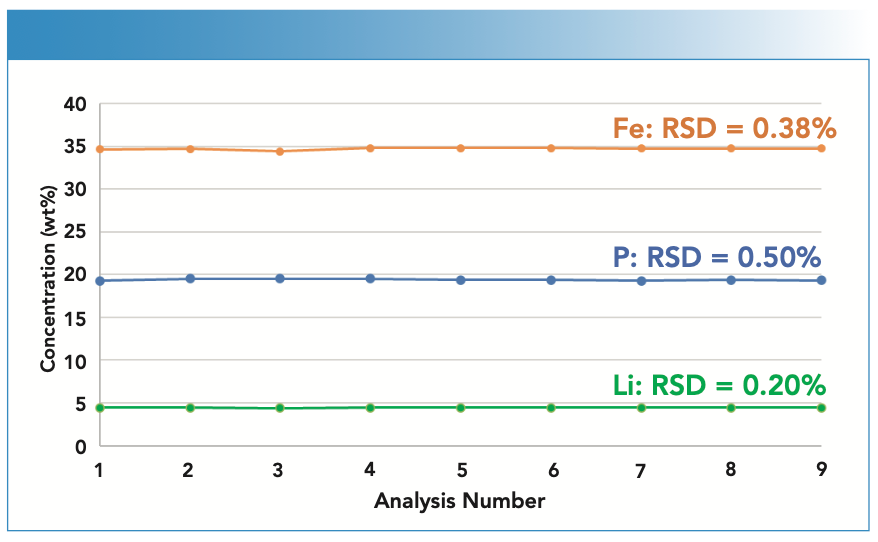
For LiFePO4, the concentration of each component in the solid is important. Similar to NMC analysis, a LiFePO4 sample was analyzed nine times over 15 min. As shown in Figure 2, the RSDs over the nine measurements were less than 0.5%, demonstrating the stability of the methodology for LiFePO4.
FIGURE 2: Concentration stability of Li, Fe, and P in an LiFePO4 sample from nine measurements over 15 min.
Battery Recycling
The black mass sample resulted from the incineration of multiple lithium-ion batteries of various types, so it is a true unknown. However, the sample is expected to contain high concentrations of common cathode elements, but there could be various cathodes used in these batteries. Additionally, a variety of impurities could also be present.
To determine what elements are present, a quick semi-quantitative screening was performed, which showed high levels of expected elements typically present in cathodes (Co, Cu, Fe, Li, Mn, Ni, and P), along with the following impurities: Al, B, S, Ti, W, and Zr. Therefore, quantitative analyses were performed on these 13 elements. The sample was analyzed two times, with the results appearing in Table IV, along with the relative percent differences between the analyses. As expected, the results show percent levels of common cathode elements (Co, Cu, Li, Mn, Ni, and P), along with lower concentrations of several impurity elements (Al, S, Ti, W, and Zr). The relative percent differences between the analyses are less than 3%, demonstrating the consistency of the methodology.
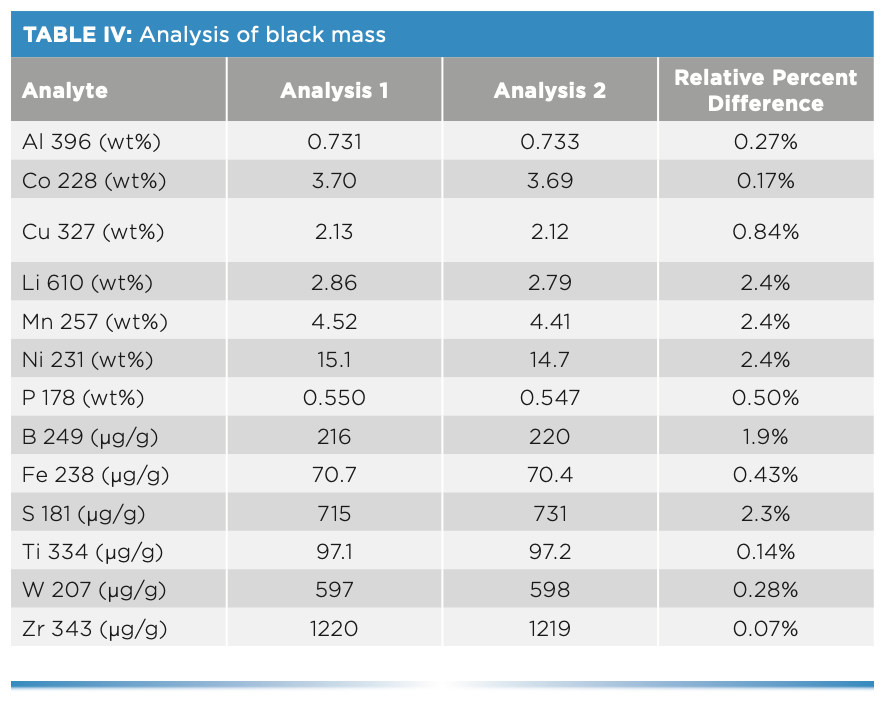
It is surprising that Al was present at percent levels as an impurity, but its presence at this concentration was confirmed through multiple sample preparations and analyses. In addition, sample prep blanks were prepared and analyzed and showed that Al was not being introduced through the sample preparation process.
Conclusion
This work has shown how ICP-OES can be used in the lithium-ion battery industry to help with the development of new battery materials and in recycling spent batteries, because of its versatility. The ability of ICP-OES to provide repeatable high-precision results allows for the accurate characterization of cathode materials, while the wide dynamic range and ability to handle challenging matrices allows it to be used in battery recycling to determine elements important for cathodes and impurity elements in combusted batteries, allowing recovery procedures to be optimized. With this versatility, ICP-OES is an important tool for the Li-ion battery industry.
References
(1) Das, D.; Manna, S.; Purvankara, S. Electrolytes, Additives and Binders for NMC Cathodes in Li-Ion Batteries—A Review. Batteries 2023, 9, 193. DOI: 10.3390/batteries9040193
(2) Azevedo, M.; Baczynska, M.; Hoffman, K.; Krauze, A. Lithium Mining: How New Production Technologies Could Fuel the Global EV Revolution; Technical Report for McKinsey & Company: London, England, April 2022.
(3) Buchert, M.; Degrief, S.; Dolega, P. Ensuring a Sustainable Supply of Raw Materials for Electric Vehicles: A Synthesis Paper on Raw Material Needs for Batteries and Fuel Cells; Technical Report for Oko-Institut: Darmstadt, Germany, 2018.
(4) PerkinElmer, HP-ICP-OES: Using the Avio 550/560 Max to Achieve the Highest Possible Precisions; Technical Note from PerkinElmer U.S. LLC: Shelton, Connecticut, 2020. https://www.perkinelmer.com/libraries/TCH_ Avio-500-ICP-OES-CRTSIS_38131 (accessed 2013-09-05).
Ken Neubauer is with PerkinElmer LLC, in Shelton, CT. Direct correspondence to: kenneth.neubauer@perkinelmer.com ●
