Study of Heavy Metals and Methyl-Mercury in Fungi in Markets of Madrid
In this study, 48 different wild and cultivated mushrooms were purchased in local markets in Madrid and provided by trusted specialists from the Mycological Society of Madrid. Cadmium, mercury, and lead were determined by inductively coupled plasma–mass spectrometry (ICP-MS) in samples previously digested in a microwave oven. Methylmercury (MeHg) was determined by liquid chromatography separation (HPLC) combined with ICP-MS after microwave-assisted extraction. Regarding the cadmium and lead contents, all the samples complied with the maximum permitted limits. For mercury, a single sample exceeded the maximum value established in Regulation (EU) 2018/73 for wild mushrooms. In the case of methylmercury, the highest amount represents 13% of the total mercury in one sample. In the rest of the cases, the concentration found was negligible.
Historically, fungi have been classified as inferior plants in the division Thalophyta because of their similarities to other members of that group (no roots, no leaves). However, later studies demonstrated unique features that led to the formation of the new kingdom, the Kingdom Myceteae, which includes microscopic and macroscopic species of fungi. Mushrooms are described as large multicellular fungi with stems and caps. According to Chang and Milles, mushrooms can be simply defined as “macro fungi with a distinctive fruiting body and large enough to be seen with the naked eye and to be picked by hand” (1).
Edible mushrooms have always been a popular food in diets around the world. Their texture and flavor make them highly appreciated in the kitchen. Consuming mushrooms brings multiple benefits because they are rich in protein, dietary fiber, vitamins and minerals, and low in fat. In addition, they contain antioxidant compounds such as polysaccharides, terpenoids, and polyphenols, all of which have a positive impact on health (2,3)
However, the scientific literature shows that mushrooms can accumulate heavy metals present in the soil. The fungal mycelium plays an essential role in this, which spreads over the available area and incorporates metal ions into its cytosol under suitable conditions. Mushrooms are able to accumulate higher concentrations than plants because, unlike their roots, their mycelium develops mainly horizontally, normally occupying the most superficial part (5–10 cm) (4). The mycelium contains the highest concentration of heavy metals (5). Although this characteristic makes them extremely useful as bioindicators of contaminated areas (6), edible mushrooms may pose a risk to consumers if the heavy metal content exceeds reference levels.
The main factors that influence the bioaccumulation of heavy metals in these organisms are environmental factors, which are the concentrations of metals in the soil, the pH, the amount of organic matter and contamination by atmospheric deposition, and intrinsic fungal factors of the species, such as its structure, biochemical composition, decomposition activity, and development of mycelium and fruit bodies (7,8).
Among the heavy metals, it is worth mentioning mercury, cadmium, and lead, which are considered toxic elements for human health by the European Food Safety Authority (EFSA).
Mercury is a toxic heavy metal that can bioaccumulate not only in marine organisms (mainly swordfish and sharks), but also in fungi. There has always been significant concern about its exposures, intakes, and toxicity. In the environment, mercury can be found in three different chemical forms: elemental/metallic mercury (Hg0), inorganic mercury (Hg+ and Hg2+), and organic mercury (organometallic compounds where one or two alkyl/aryl substituents are attached to the mercury atom). These forms present different bioavailabilities and toxicity, with methylmercury being the most dangerous in the food chain (9).
To perform an accurate risk assessment, it is necessary to collect as much information as possible on the presence of these forms in food because exposure to methylmercury can cause harm by interacting with the sulfhydryl (-SH) groups distributed throughout the body, causing the well- known Minamata disease (6).
The central nervous system is the most important target of methylmercury, which can cause different symptoms, including sensory disturbances in the hands and feet, ataxia, and visual impairment. In addition, organic mercury can reduce the activity of natural killer (NK) cells and generate free radicals. Acute inorganic mercury poisoning affects the gastrointestinal tract and kidneys, causing severe diarrhea, vomiting, and renal tubular necrosis with anuria (6).
In the 2012 EFSA opinion, the tolerable weekly intake (TLWI) for methylmercury was updated to 1.3 μg/kg bw and 4.0 μg/kg bw for inorganic mercury, which is the maximum amount that a person can ingest weekly throughout their lives without adverse effects (9).
European legislation establishes maximum Hg contents in mushrooms. Commission Regulation (EU) 2018/73 distinguishes between cultivated and wild mushrooms, establishing maximum limits of 0.050 mg/kg and 0.50 mg/kg of Hg, respectively. It also establishes a maximum limit of 0.90 mg/kg in Boletus spp. (10).
Cadmium is also a heavy metal that poses serious risks to human health. Until recently, it has not been possible to demonstrate that cadmium has any physiological function, so its main interest lies in its high toxicity in humans, especially in its inorganic forms (11).
Various studies have shown that exposure to cadmium through diet can cause harmful effects on health, mainly in the kidney, spleen, and liver where it accumulates (12). In addition, it is currently classified by the International Agency for Research on Cancer (IARC) as carcinogenic to humans (Group 1), as an association between its intake and the risk of postmenopausal endometrial cancer has been demonstrated. As a result of its ability to induce oxidative stress, it can also affect male fertility by decreasing sperm quality (13).
Prolonged ingestion of cadmium also poses a serious health risk, by causing Itai-Itai disease, the best-known case of which was the mass poisoning that took place in Japan in the last century. This disease is characterized by creating bone damage due to osteoporosis and osteomalacia, in addition to severe kidney damage (14,15). Derived from a comprehensive toxicological assessment, EFSA has established a tolerable weekly intake (TSI) for cadmium of 2.5 μg/kg body weight (16).
In 2023, the maximum cadmium content in mushrooms has been updated by Regulation (EC) No 2023/915, establishing the maximum limit at 0.050 mg/kg for cultivated mushrooms, at 0.15 mg/kg for the mushrooms Lentinula edodes (shiitake mushroom) and Pleurotus ostreatus (oyster mushroom), and 0.50 mg/kg in wild mushrooms (17).
Finally, lead is another heavy metal to consider. Lead contamination arises primarily from the environment, through atmospheric lead through deposition on agricultural crops, from water, or from food processing, handling, and packaging. Although lead exists in both organic and inorganic forms, only inorganic lead has been detected in food (18). When lead is ingested, it is distributed to certain organs such as the brain, kidneys, liver, and bones, causing tissue damage. After several weeks, most of it moves to the bones and teeth, where it can be stored for decades (19,20).
Accumulated lead can return to the blood, reaching vital organs, resulting in episodes of broken bones in the elderly. During pregnancy, accumulation of lead can lead to possible effects on the fetus and lactation (20).
Lead poisoning produces adverse effects at the central nervous system level, which can affect brain development and cause behavioral changes such as reduced attention span and increased antisocial behavior. In addition, it can lead to anemia, hypertension, renal failure, immunotoxicity, and toxicity to reproductive organs (19).
Due to the lack of sufficient scientific information, in 2010 the existing toxicological safety threshold for lead was withdrawn, and a new one could not be determined. Therefore, there is currently no recommended tolerable intake for lead (21).
As of this year, the maximum lead content in mushrooms has been updated by Regulation (EC) No. 2023/915, establishing the maximum limit at 0.30 mg/kg for Agaricus bisporus (mushroom), Pleurotus ostreatus (oyster mushroom) and Lentinula edodes (shiitake mushroom) mushrooms, and 0.80 mg/kg in wild mushrooms (17).
The main objective for this study is to provide information on the content of cadmium, lead, mercury, and methylmercury in both cultivated and wild mushrooms and to compare the results obtained with the limits established in current legislation. For this, a total of 48 mushrooms were obtained and analyzed.
Materials and Methods
Equipment and Operating Conditions
For the analysis of cadmium, lead, and total mercury, the inductively coupled plasma mass spectrometer (ICP-MS) (Agilent Technologies model 7900) was used. In addition, an integrated sample introduction system (ISIS 3) and an automatic sampler (SPS 4) were used in the determination together with the ICP-MS. The operating parameters of the ICP-MS analysis are shown in Table I.
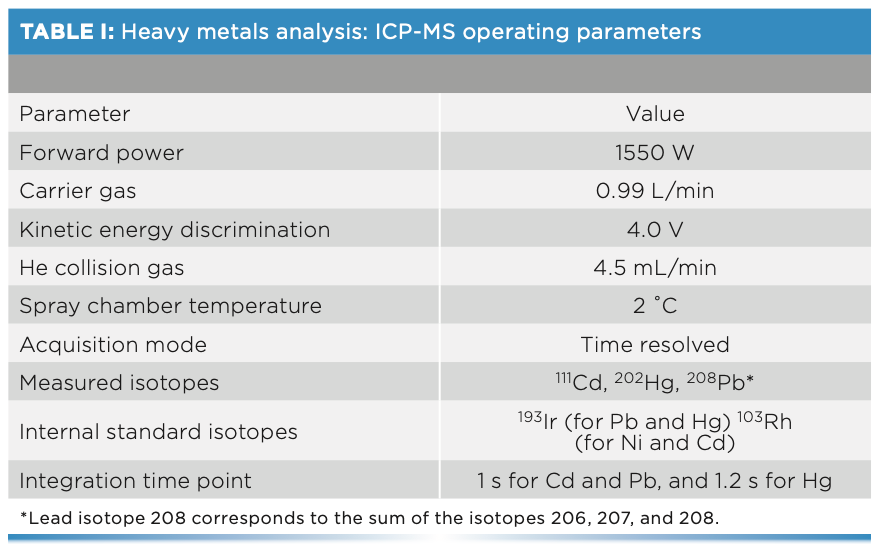
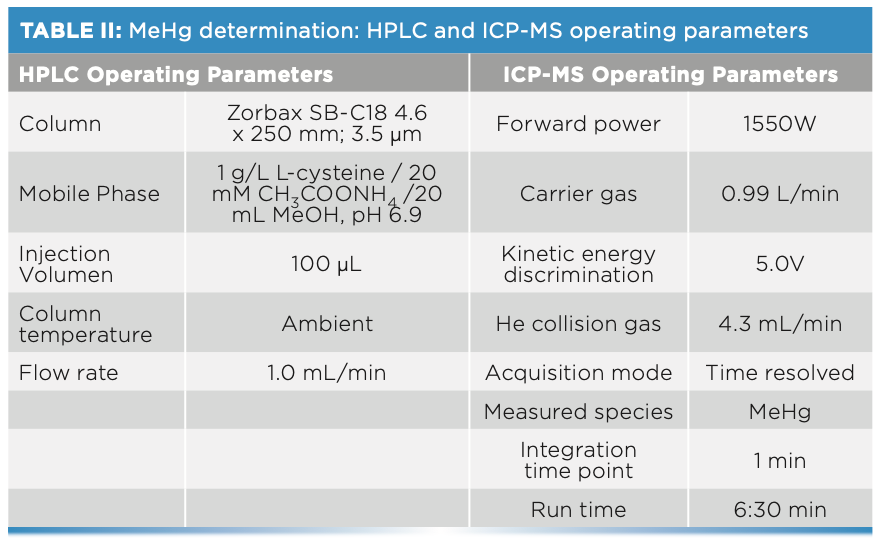
Methyl-mercury determinations were performed using a liquid chromato- graph (HPLC) (Agilent Technologies 1200 Infinity), consisting of a model 1260 Quaternary Pump VL, a model 1260 ALS injector, and a model 1260 TCC column compartment. This HPLC system is coupled to the ICP-MS described above. The characteristics are detailed in Table II.
For digestions and extractions, a DigiPREP Jr digestion block from SCP Science with 24 positions was used. The volumetric material used for all the solutions were DigiTubes, polypropylene tubes single-use, except in the case of MeHg analysis, which opted for centrifuge tubes, also single-use, VWR Metal Free, 15 mL, and volumetric flasks of 1 L and 200 mL capacity. To take the corresponding aliquots, Eppendorf brand micropipettes were used.
For centrifugation of the samples, a Lisa refrigerated centrifuge of AFI groups was used.
Reagents and Standards
For the preparation of the reagents, dilutions, washing, and rinsing solution of the work material, deionized water (H2O) of Class Type I (according to the UNE-EN-ISO 3696 Standard) was used, purified with the Milli-Merck’s Q Comprehensive. Nitric acid (HNO3, 65%) from Scharlau was distilled in the laboratory using Savillex equipment, model DST-1000. Suprapur hydrochloric acid for trace analysis (HCl, 37%) supplied by Scharlau and hydrogen peroxide (H2O2) used was Merck, 30%, Suprapur.
Regarding the ICP-MS, the tuning solution containing 1 μg/l Ce, Co, Li, Mg, Tl, and Y was used. Finally, argon and helium (99.999%) for plasma generation and interference removal.
Standards of 1000 mg/L of Cd (Scharlau), Pb (VHG-Labs), Hg (Scharlau), and MeHgCl (VHG-Labs) were also used. For the determination of cadmium, lead, and total mercury, the intermediate solutions and the calibration curve were prepared with a 2% HNO3 and 1% HCl (v/v) solution.
As an internal standard, a 0.4 mg/L iridium and rhodium solution (from a 1000 mg/L Scharlau standard) was prepared in a 2% HNO3, 1% HCl, and 5% acetic acid solution for the determination of cadmium, lead, and total mercury. To stabilize the signals, 0.4 mg/L Au from SCP Science was added. No internal standard was used for the methylmercury speciation method.
The composition of the mobile phase was 1 g/L L-cysteine/20 mM CH3COONH4/20 mL MeOH in deionized water, with a pH of 6.9, using L-cysteine from Acros Organics (minimum purity 98%), ammonium acetate (CH3COONH4) from PanReac AppliChem (96% purity), and methanol (MeOH) from Scharlau (GC residue analysis grade, d = 0.79 g/cm3).
In the case of the mercury speciation analysis, the solution used for the extraction was 5 g/L L-cysteine/0.5% HCl, and the solution used for the preparation of all the intermediate solutions and the calibration curve was the mobile phase.
Sample Treatment and Extraction Method
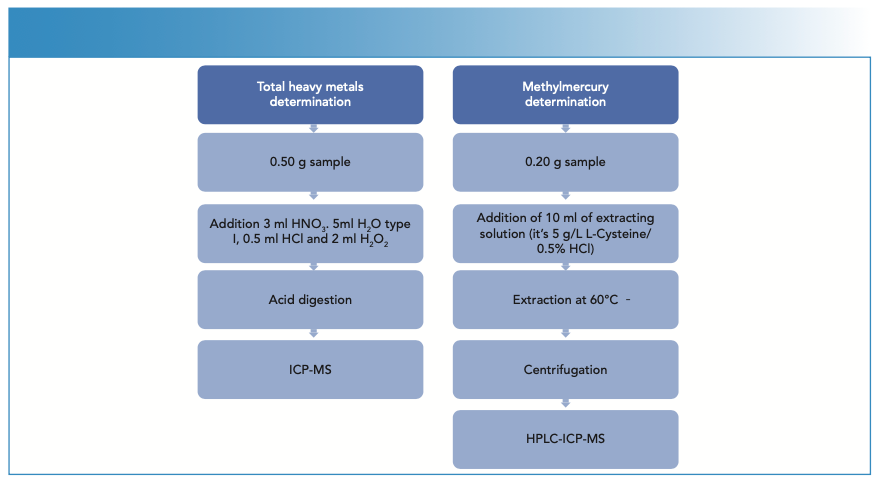
The samples were homogenized by crushing with a mincer, until a representative sample of the product to be tested was obtained. Two different methods were carried out in this study. Both represented in Figure 1.
FIGURE 1: Schematic diagrams of the different methods (total heavy metals and methylmercury) used for this study.
Determination of Heavy Metals
Regarding the total analysis of mercury, cadmium, and lead, first 0.5 ± 0.05 g of sample were weighed in a DigiTube, then 3 mL HNO3, 5 mL H2O, 0.5 mL HCl, and 2 mL H2O2 were added, and the samples were digested in the DigiPrep afterwards. Once the digestion was complete and the samples had reached room temperature, they were made up to volume in 50 mL with deionized water. The sample collection were analyzed by ICP-MS, samples with high analyte contents that were diluted previously.
Determination of Methylmercury
For methylmercury speciation, 0.2 ± 0.02 g of sample was weighed into a DigiTube, 10 mL of extraction solution (1 g L-cysteine; 5% HCl) was added, and extraction was performed on the DigiPrep for 30 min at 60 oC (Figure 2). Once the process was finished, the samples were allowed to cool to room temperature, transferred to 15 mL tubes, and centrifuged for 10 min at ≥ 4000 rpm and 4 oC. Subsequently, the supernatant was filtered using 0.45 μm pore size filters and placed in 1-mL polypropylene vials to be injected into the HPLC system.
FIGURE 2: Temperature profile for methylmercury speciation. For sample preparation, 0.2 ± 0.02 g of the sample was weighed into a sample tube, 10 mL of extraction solution (1g /L-cysteine; 5% HCl) was added, and extraction was performed for 30 min at 60 °C.
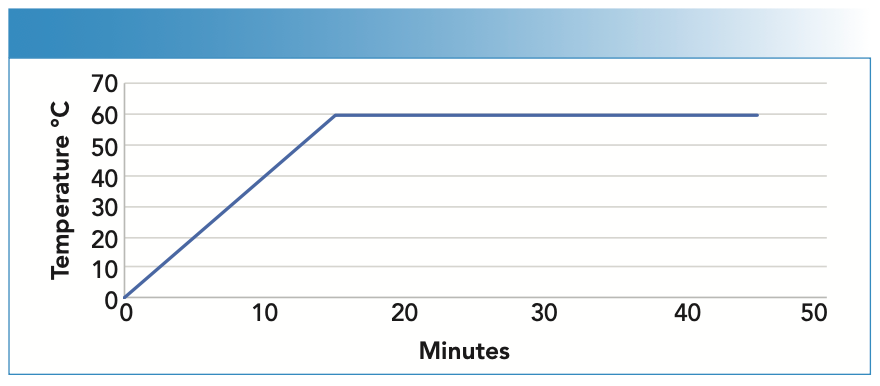
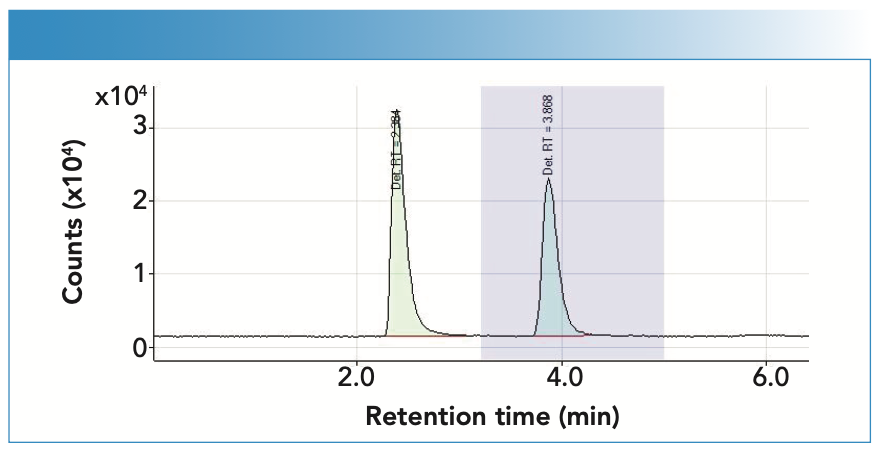
Figure 3 shows a chromatogram where you can see the separation of the peaks obtained, where the first corresponds to Hg, with a retention time of 2.384 min, and the second to MeHg, with a retention time of 3.868 min.
FIGURE 3: The chromatogram illustrates the separation of the peaks obtained, where the first corresponds to Hg, with a retention time of 2.384 min, and the second to MeHg, with a retention time of 3.868 min.
Calibration Curves
For the calibration curves used in the quantification of the analytes, the y-axis represents the value of the ratio, calculated as the number of counts of the analyte divided by the number of counts of the internal standard, whereas the x-axis represents the concentration in μg/L. In the case of MeHg, the y-axis represents the number of counts per area of the chromatographic peak.
The calibration curves are of the type y = mx + n; where m is the slope and n is the ordinate at the origin.
Figures 4, 5, 6, and 7 show the calibration curves used in the quantification of the samples.
FIGURE 4: Calibration curve (with statistics) used in the quantification of cadmium (Cd).
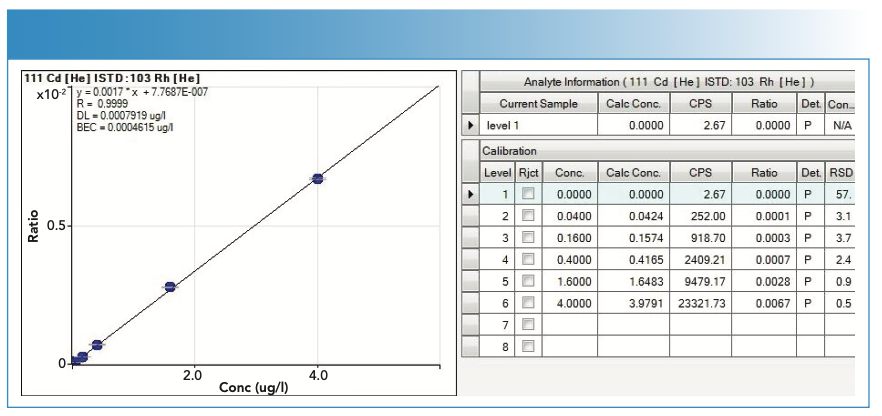
FIGURE 5: Calibration curve (with statistics) used in the quantification of lead (Pb).
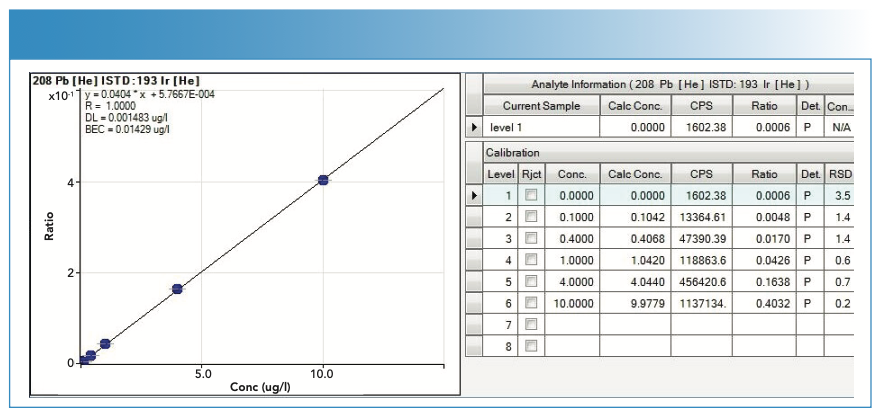
FIGURE 6: Calibration curve with statistics used in the quantification of mercury (Hg).
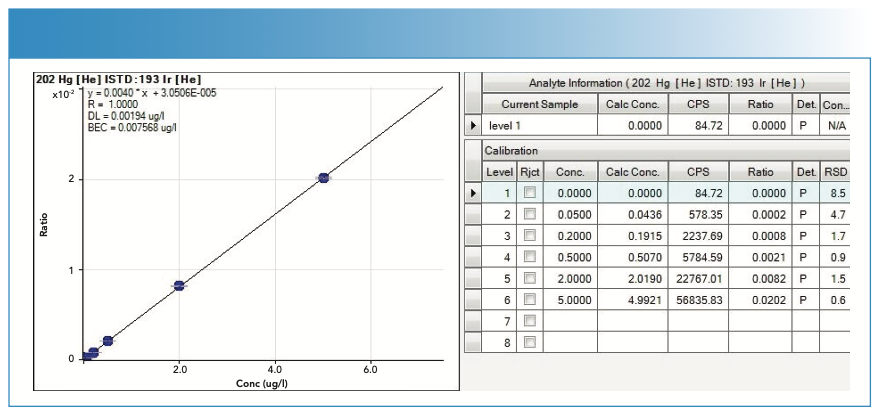
FIGURE 7: Calibration curve with statistics used in the quantification of methylmercury (MeHg).

Validation and Quality Control
The test method used for the determination of heavy metals meets the criteria of Regulation (EC) 333/2007 of the Commission of March 28, 2007, which establishes the sampling and analysis methods for the control of levels trace elements and process contaminants from food products and their modifications. The validation was carried out according to the requirements of the UNE/EN-ISO 17025 Standard for Testing Laboratories, and it is accredited by the National Accreditation Entity (ENAC). For MeHg determination, the test method is in the accreditation process.
Results
A total of 48 wild and cultivated mushrooms were acquired and analyzed. All of them were purchased in local markets in Madrid and provided by trusted specialists in the manual collection of mushrooms from the Mycological Society of Madrid.
The results obtained in the study of cadmium, lead, and total mercury of the 48 samples analyzed are shown in Table III.
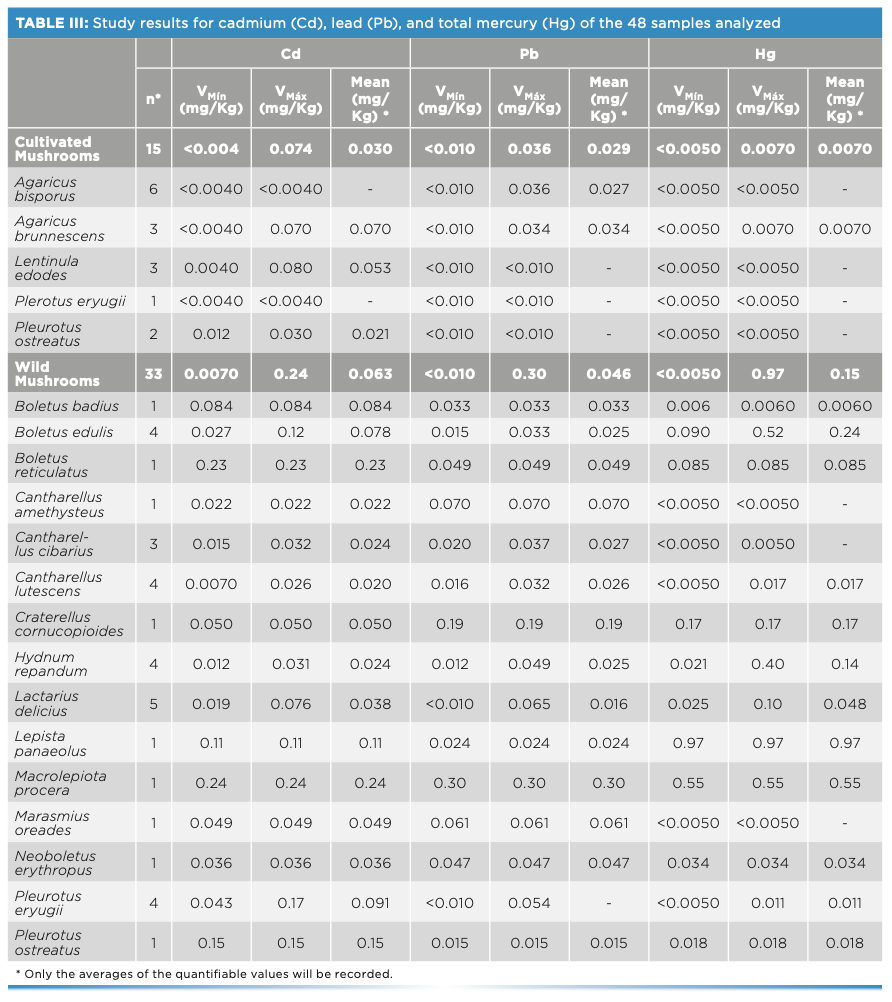
Regarding the cadmium and lead contents, all the samples comply with the maximum permitted limits, and do not seem to pose a risk to the population.
Regarding the total mercury content, it should be noted that a single sample (Lepista luscina with a content of 0.97 mg/kg) exceeded the maximum value established in Regulation (EU) 2018/73, set at 0.50 mg/kg for wild mushrooms. No sample of Boletus spp. reached the maximum allowed limit of 0.90 mg/kg for said species. In the case of samples of cultivated mushrooms, it is observed that their Hg content is less than 0.050 mg/kg established by the aforementioned regulation.
According to the data obtained, it can be observed that cultivated mushrooms have significantly lower concentrations of metals than wild mushrooms.
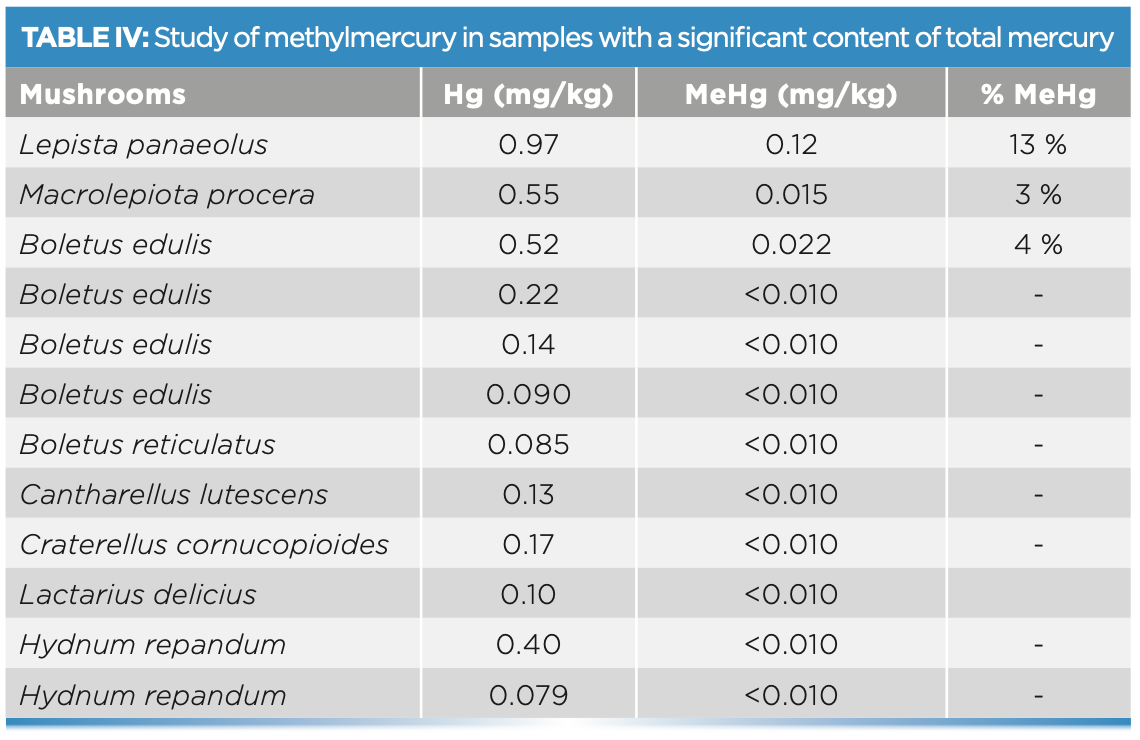
The results of the methylmercury determination are shown in Table IV. In this case, only the samples with a significant total mercury content were analyzed. Speciation was performed in the 10 samples that exceeded 0.075 mg/kg of total Hg, all of them wild. The highest amount of MeHg found was 0.12 mg/kg in a sample of Lepista panaeolus, which represents 13% of the total mercury in the sample.
Conclusion
It can be determined that the test method used for the analysis of Cd, Hg, and Pb by acid digestion and determination by inductively coupled plasma–mass spectrometry is considered a valid option to carry out a study, such as the one presented here, obtaining results in accordance with the requirements of ISO 17025.
In the same way, the coupling of a liquid chromatograph to an ICP-MS is a recommended method to perform the chromatographic separation of the different mercury species, and to be able to achieve the methylmercury analysis. This method is in the accreditation process.
After determining the content of cadmium, lead, and total mercury and the speciation of methylmercury in the samples analyzed, it can be concluded that the amounts found in the case of cultivated mushrooms are lower than the limits established in European legislation, whereas in the case of wild mushrooms, a result was obtained above the established limits, which may mean a risk to the population.
The values obtained by performing the mercury speciation reveal that the mercury content in the mushrooms is almost entirely inorganic mercury and the amounts of methyl mercury found are practically negligible, becoming, in the worst case, less than 13% of total Hg.
Acknowledgments
We would like to thank the Mycological Society of Madrid for its invaluable role in providing us with samples of wild mushrooms in order to develop the study of their metal content.
References
(1) Chang, S.T.; Miles, P. G. Mushroom: Cultivation, Nutritional Value, Medicinal Effect, and Environmental Impact; CRC Press, 2004.
(2) Gupta, S.; Summuna, B.; Gupta, M.; Annepu, S. K. Bioactive Molecules in Food; Springer International Publishing AG, 2018; pp 1815–1847.
(3) Heleno, S. A.; Barros, L.; Martins, A.; et al. Chemical Composition, Antioxidant Activity, and Bioaccessibility Studies in Phenolic Extracts of Two Hericium Wild Edible Species. LWT 2015, 63, 799–806.
(4) Allen, M. F. The Ecology of Mycorrhizae; Cambridge University Press, 1991.
(5) Berthelsen, B. O.; Steinnes, E. Accumulation Patterns of Heavy Metals in Soil Profiles as Affected by Forest Clear-Cutting. Geoderma 1995, 66, 1–14. DOI: 10.1016/0016-7061(94)00072-I
(6) Bernhoft, R. A. Mercury Toxicity and Treatment: A Review of the Literature. J. Environ. Public Health. 2012, 460508. DOI: 10.1155/2012/460508
(7) García, M. A.; Alonso, J.; Fernández, M. I.; Melga, M. J. Lead Content in Edible Wild Mushrooms in Northwest Spain as Indicator of Environmental Contamination. Arch. Environ. Contam. Toxicol. 1998, 34, 330–335.
(8) Lalotra, P.; Gupta, D.; Yangdol, R.; Sharma, Y. P.; Gupta, S. K. Bioaccumulation of Heavy Metals in the Sporocarps of Some Wild Mushrooms. Curr. Res. Environ. Appl. Mycol. 2016, 6, 159–165. DOI: 10.5943/cream/6/3/2
(9) European Food Safety Authority. EFSA Journal 2012, 10 (12), 2985. https://www.efsa.europa.eu/en/efsajournal/pub/2985
(10) European Commission. Commission Regulation (EU) 2018/73 of 16 January 2018 Amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as Regards Maximum Residue Levels for Mercury Compounds in or on Certain Products. https://eur-lex.europa.eu/collection/eu-law/consleg.html
(11) Godt, J.; Scheidig, F.; Grosse-Siestrup, C.; et al. The Toxicity of Cadmium and Resulting Hazards for Human Health. J. Occup. Med. Toxicol. 2006, 1, 22. DOI: 10.1186/1745-6673-1-22
(12) Kalač, P.; Svoboda, L.; Havlíčková, B. Contents of Cadmium and Mercury in Edible Mushrooms. J. Appl. Biomed. 2004, 2, 15–20. DOI: 10.32725/jab.2004.002
(13) Ciobanu, C.; Şlencu, B. G.; Cuciureanu, R. Estimation of Dietary Intake of Cadmium and Lead through Food Consumption. Rev. Med. Chir. Soc. Med. Nat., Iaşi 2012, 116 (2), 617–623.
(14) Satarug, S. Dietary Cadmium Intake and Its Effects on Kidneys. Toxics 2018, 6 (1), 15. DOI: 10.3390/toxics6010015.
(15) Inaba, T.; Kobayashi, E.; Suwazono, Y.; et al. Estimation of Cumulative Cadmium Intake Causing Itai–Itai Disease. Toxicol. Lett. 2005, 159, 192–201. DOI: 10.1016/j.toxlet.2005.05.011
(16) Cadmium in Food—Scientific Opinion of the Panel on Contaminants in the Food Chain. EFSA Journal 2009, 980, 1–139. DOI: 10.2903/j.efsa.2009.980
(17) European Commission. Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No. 1881/2006. https://eurlex.europa.eu/homepage.html
(18) World Health Organization. Evaluation of Certain Food Additives and Contaminants: Seventy-Third Report of the Joint FAO/WHO Expert Committee on Food Additives; WHO Technical Report Series; 960; Geneva, Switzerland, 2011.
(19) World Health Organization. Lead Poisoning and Health. 2022. https://www.who.int/news-room/fact-sheets/detail/lead-poisoning-and-health
(20) Agency for Toxic Substances and Disease. Public Health Statement Lead CAS# 7439-92-1. https://wwwn.cdc.gov/TSP/PHS/PHS. aspx?phsid=92&toxid=22, 2007
(21) Scientific Opinion on Lead in Food. EFSA Journal 2010, 8 (4), 1570. DOI: 10.2903/j.efsa.2010.1570
Javier Peinador Asensio is with the Public Health Laboratory, in Madrid, Spain. Direct correspondence to: peinadoraj@madrid.es ●
High-Speed Laser MS for Precise, Prep-Free Environmental Particle Tracking
April 21st 2025Scientists at Oak Ridge National Laboratory have demonstrated that a fast, laser-based mass spectrometry method—LA-ICP-TOF-MS—can accurately detect and identify airborne environmental particles, including toxic metal particles like ruthenium, without the need for complex sample preparation. The work offers a breakthrough in rapid, high-resolution analysis of environmental pollutants.
Trending on Spectroscopy: The Top Content of 2024
December 30th 2024In 2024, we launched multiple content series, covered major conferences, presented two awards, and continued our monthly Analytically Speaking episodes. Below, you'll find a selection of the most popular content from Spectroscopy over the past year.
