Assignment of Raman Bands of a Set of Biopolymers with Small Increases in an Added Functional Group
Raman spectra were measured in combination with 2D-COS analysis to understand how the addition of propyl side groups to a biopolymer backbone influences the structure of the polymer at the atomic level.
We recorded the Raman spectra of NodaxTM (PHBHx-copolymer of hydroxybutyrate) biopolymer class with varying amounts of comonomer hydroxyhexanoate (Hx). The properties of Nodax is controlled by the amount of Hx added. Engineering the polymer for specific applications requires understanding the effects of Hx on the physical and chemical properties, and vibrational spectra provide a window on this information. Inspection of the structure of PHBHx indicates that the addition of Hx only replaces the methyl group with a propyl group on the hydroxyhexanoate segments of the polymer chains. To utilize the vibrational spectra to aid in the prediction of properties, the bands associated with these extra groups should be identified. The spectra shown here were acquired from polymers dissolved in deuterated chloroform; the carbon–hydrogen band of the solvent that would normally overlap with the spectrum of the polymer is replaced by a carbon-deuterium (CD) stretch in a region in which nothing else occurs. In solution, the spectra of the polymers exhibit the noncrystalline disordered phase and can be used to evaluate the contributions of the various functional groups.
The polymers discussed here were originally engineered by Professor Isao Noda during his career at Proctor & Gamble (P&G). The goal was to engineer a class of polymers that would be biodegradable, renewable, and with “attributes including polyolefin-like thermomechanical properties, polyester-like physicochemical properties, and interesting biological properties” (1). The structure of the polymers that we discuss is shown schematically in Figure 1.
FIGURE 1: Structure of Nodax. The polymeric unit on the right (in parentheses) is polyhydroxybutanate (PHB), while the monomer on the left (in parentheses) appears with the incorporation of hydroxyhexanoate in the chain.
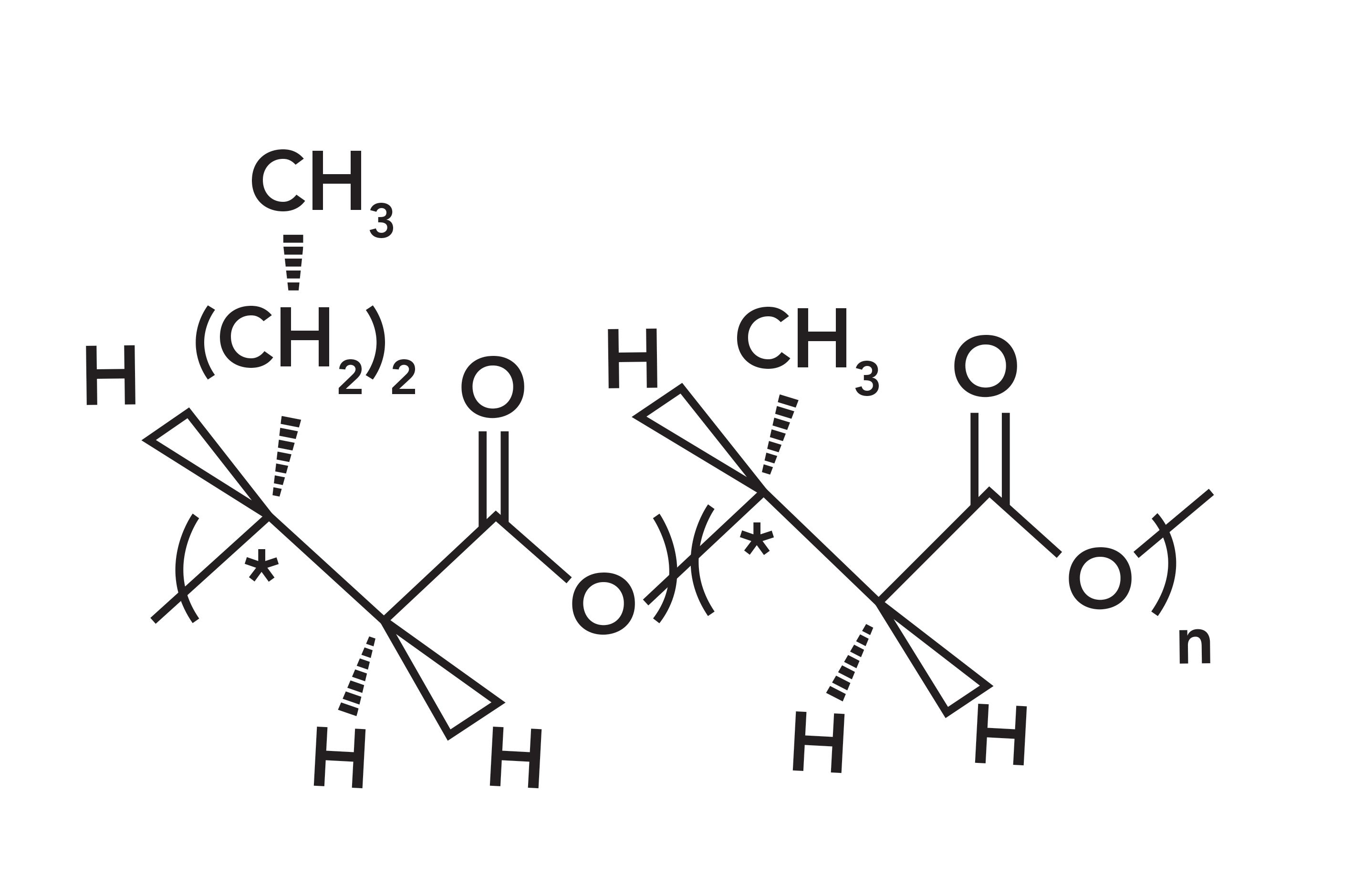
A pure polyhydroxybutyrate (PHB) homopolymer would contain only the monomer shown on the right of Figure 1. The pure PHB is quite crystalline and difficult to melt or dissolve. When small amounts of hydroxyhexanoate (shown on the left of the figure) is introduced as isolated monomers in the chain, the result is a random copolymer, and those side chains inhibit crystallization, which is excessive in PHB. The importance of controlling the degree of crystallinity is that it provides control of the physical and chemical properties. Polymer manufacturing is done in a bioreactor fermentation process by soil bacteria, and control of the side chains is provided by feeding appropriate fatty acids under specific conditions. Because the entire polymerization process is enzymatically controlled, the configuration of all the carbons are defined.
Figure 1 shows that the polymer segment on the right is formed from the monomer hydroxybutyrate, while the monomer on the left is formed during polymerization with hydroxyhexanoate. Regardless of the side chain, each monomeric unit has one carbonyl group, one methyl group in the chain, one methine proton in the chain, and either a methyl or propyl side group next to the ester linkage. Because the substituted amount is typically less than one in seven, the likelihood of these “extra” monomers being nearest neighbors is small, so we consider the polymer a random rather than block co-polymer. It is not difficult to understand that the presence of the side groups which appear occasionally along the chain, interrupts the regularity and thus prevents crystallization.
These polymers are biodegradable either aerobically or anaerobically, with rates comparable to that of cellulose. Products made, tested, and illustrated in the cited article include dinnerware, detergent bottles, clear bags, and fibers (1). The development of these materials has enabled their commercialization by Danimer Scientific, Inc.
Use of Vibrational Spectroscopy in Analyzing Nodax
Vibrational spectra encode information on functional groups: their bond strength, bond order and angles, and interactions between groups that are geometrically close. For small molecules, calculations are straightforward in predicting spectra. For polymers, the situation is complicated because of the size of the molecule and the orientation of the backbone regardless of whether it is crystalline; the latter two properties can affect interactions between groups that change with polymer history. But when vibrational spectra can be interpreted, it has the potential to aid in the prediction of physical and chemical properties.
In this study, we focused on confirming the assignments of the bands in the carbon–hydrogen region to determine if extra bands of the extra methylene on the side chain could be differentiated from the methylene on the backbone, and whether the methyl group on the backbone could be differentiated from the methyl at the end of the side chain. This information will be helpful in understanding the crystallization process.
Description of Samples and Measurement Protocol
Samples of PHBHx with substitutional percentages of hexanoate of 0, 3.8, 5.8, 6.2, 7.6, 9.4, and 13% were examined. Small amounts of the samples were dissolved in deuterated chloroform (CDCl3) which enabled us to record spectra of isolated molecules in solution, without interference from the carbon–hydrogen band of the solvent. Except for the 0% sample, all samples dissolved without any intervention. The 0% sample was dissolved by heating it while stirring, and even then only a small amount of material went into solution, so there is significantly more noise in the spectrum of this sample than the others. First, the intensity of the carbon-deuterium (CD) band at 2248 cm-1 of the solvent in all the spectra was normalized to the spectrum of the neat solvent so the contributions of the solvent could be subtracted. After subtracting the solvent spectrum, and normalizing to the intensity of the carbonyl band whose concentration does not change with composition, the behavior in the carbon–hydrogen region can be analyzed by multiplicative curve resolution (MCR) and two-dimensional correlation spectroscopy (2D-COS). In all cases, a broadening of the solvent spectrum on the high frequency side was observed, and this created an artifact in the subtraction; however, because there is nothing of interest in this region of the spectrum, it did not interfere with the analysis. After the solvent contributions were subtracted, the spectra of all the samples were scaled so that the carbonyl band has the same intensity in all the spectra. This pretreatment enables assigning any spectral changes to the replacement of the methyl side group by the propyl side group.
Results
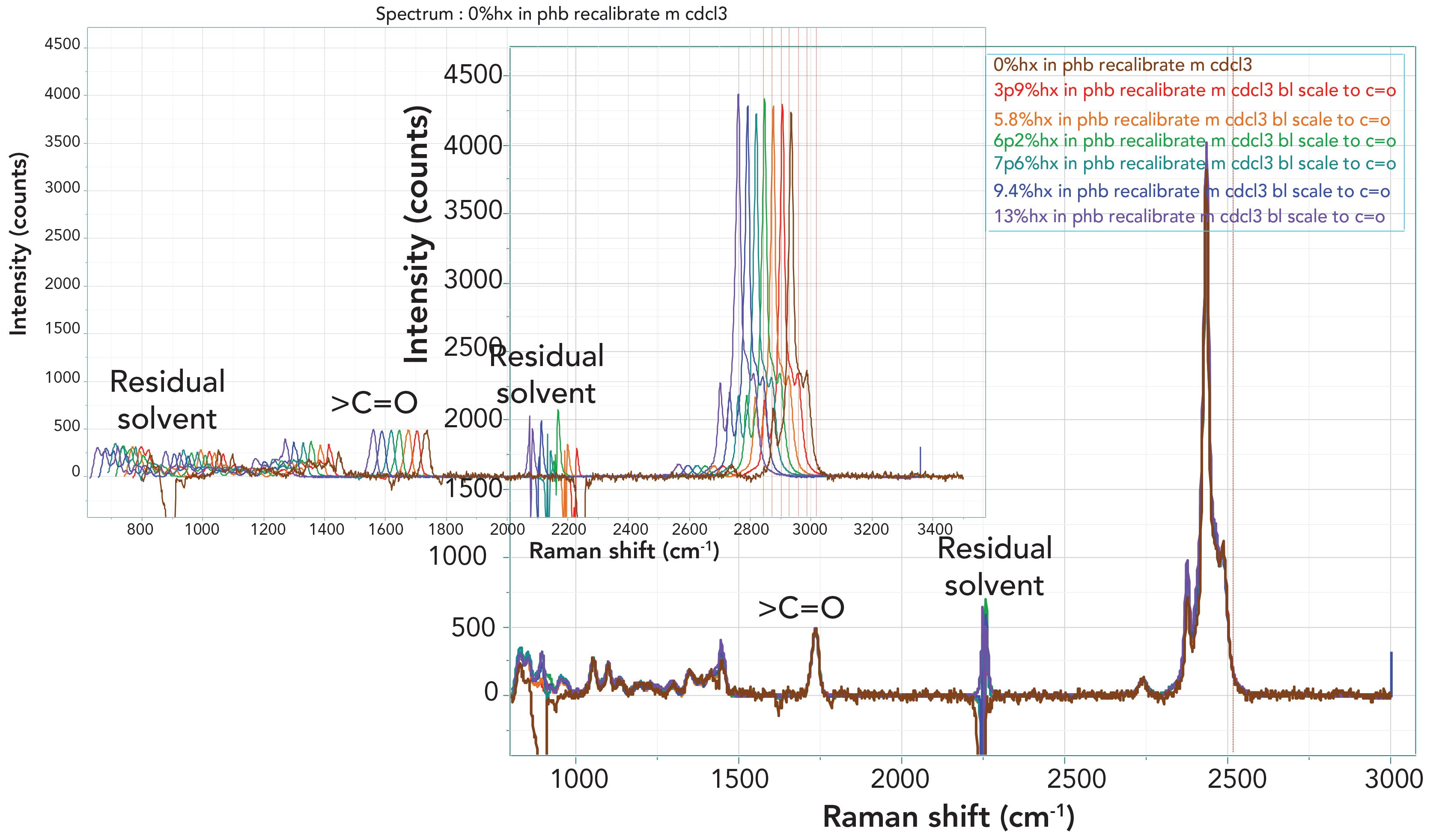
Figure 2 shows the spectra overlaid by two different methods. The lower plot shows the spectra overlaid exactly, and little detail can be seen. In the upper plot, the different samples have been displaced a bit horizontally to determine if subtle differences can be observed. Note that color coding has been used to visualize the increasing hydroxhexanoate (Hx) in the composition (brown to purple).
FIGURE 2: Full Raman spectra of all seven Nodax samples after subtraction of the CDCl3 solvent spectrum and scaling to the carbonyl band. On the bottom right, the spectra are exactly overlaid. On the top left, the spectra are displaced for better comparison.
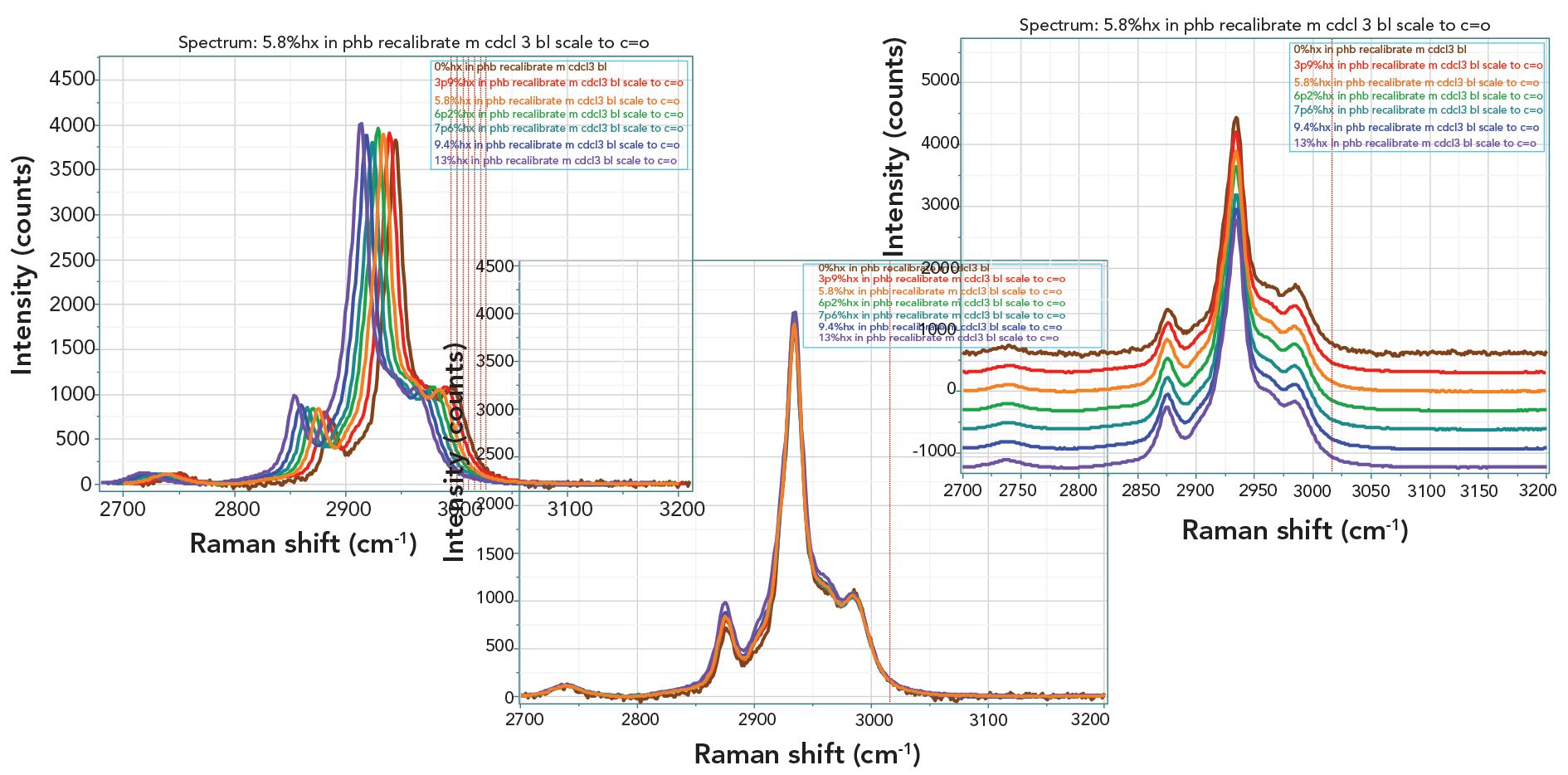
For the purposes of this study, we were most interested in the carbon–hydrogen region after the solvent subtraction and normalization had been completed. Figure 3 shows the spectra in this region, displayed now in three different ways in order to “see” the changes. Inspection of these spectra shows changes, but they are so subtle that drawing conclusions would be suspect.
FIGURE 3: Raman spectra of the seven PHBHx samples in the carbon–hydrogen region after subtraction of the solvent signal and normalizing to the carbonyl band whose concentration is constant throughout the sample set.
In the literature, there is a great deal of information on the assignment of the carbon–hydrogen bands to symmetric and asymmetric methylene stretches, symmetric and asymmetric methyl stretches, and the methine stretch. In general, as the vibrational frequency increases for a given compound, the bands appear in the order of symmetric methylene stretch, symmetric methyl stretch, asymmetric methylene stretch, and asymmetric methyl stretch. However, the range of frequencies in different compounds is quite large, so we did not assign these bands in the spectra. Rather, we employed two mathematical methods, 2D-COS and multiplicative curve resolution (MCR), to analyze the spectra. The results are shown in Figure 4.
On the left, we have displayed the results of the 2D-COS analysis of these spectra. Recall that the synchronous plot on the left indicates which bands are changing simultaneously. In these materials, it is the increase in propyl side groups that differentiate the samples, so we would expect that any changes would all be in the same direction, which is what the plot shows.
FIGURE 4: (a) 2D-COS analysis of the seven spectra in the carbon–hydrogen region and (b) Two multiplicative curve resolution (MCR) factors displayed together with their spectrum.
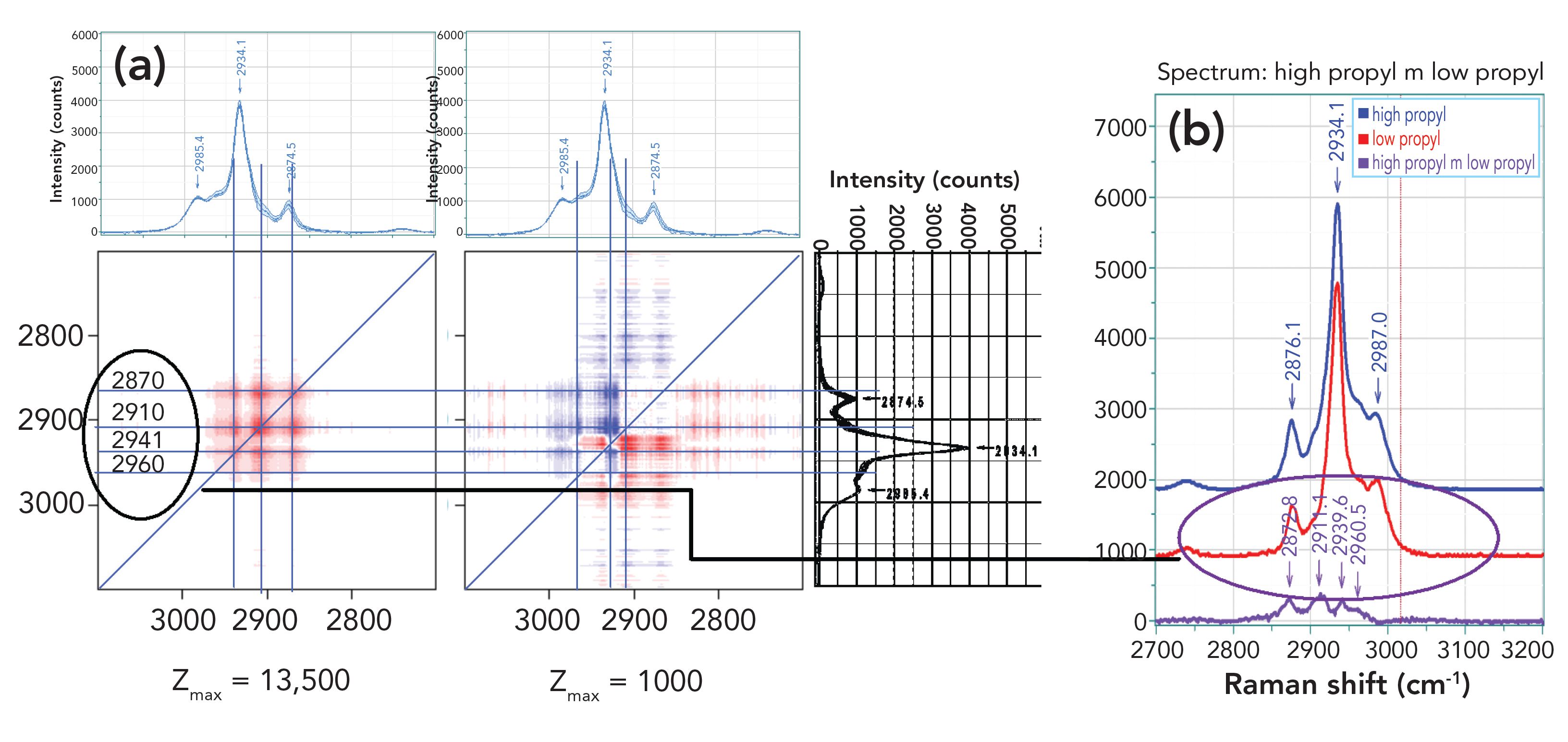
The right hand plot of the 2D-COS analysis shows the asynchronous changes. It is important to understand that if all of the changes are in phase (that is, they occur simultaneously), then the asynchronous plot will be blank. As a result, the changes in the asynchronous plot will indicate what is changing before what. I have drawn horizontal and vertical lines to evaluate where the intensity peaks are occurring relative to the spectra that are displayed above and to the right of the 2D-COS plots. In the asynchronous plot, I have lines at 2870, 2910, 2941, and 2960 cm-1, with these frequencies read from the 2D Shige software. These positions do not correspond to the highest intensity peaks in the spectra, and if you look carefully at the spectra at the bottom center of Figure 3, you can see that the changes are actually occurring between the largest peaks, approximately at the positions noted in the 2D-COS plots. Looking now at the plots on the right of Figure 4, you can see the results of the MCR analysis; in fact, the two factors plotted this way cannot be easily differentiated. But the interesting part is that when I calculated the difference between these two spectral factors, I see four little peaks that line up fairly closely to the fiduciary lines in the 2D-COS plots. My initial reaction was “Wow!” because the two mathematical manipulations are performing calculations on the same data set, the results should be consistent. But my understanding of these two mathematical algorithms is that they are different enough that the fact that the results are consistent means that the results reflect the reality of the changes. As a result, we have changes at the wavenumber positions noted earlier and shown in the figure.
As noted earlier, general assignments (in increasing vibrational frequency) appear as methylene symmetric stretch, methyl symmetric stretch, methylene asymmetric stretch and methyl asymmetric stretch. If we take these assignments to be relevant to this system, and then look at the asynchronous plot, we can start to determine how the addition of the methylene group on the side chain affects perhaps the geometrical positioning of the methyl group on the backbone against the methyl group that has moved father from the backbone. For instance, the asynchronous plot has spots of opposite color at ~ (2910, 2940) and ~ (2940, 2910). We tentatively assigned this to a methyl symmetric stretch and a methylene asymmetric stretch. Then, the change (increase) in the methylene comes before the change in the methyl because of the placement of the methyl farther from the backbone.
Concluding Remarks
When Professor Noda began developing the Nodax polymer during his work at P&G, he realized that he needed a robust way to analyze the properties of the materials that he was making. This was the impetus to develop 2D-COS for analysis of vibrational spectra. When one tries to analyze spectra using band-fitting algorithms, it quickly becomes evident that the “fits” are not unique, which means that any conclusions that one draws will be subject to some uncertainty. On the other hand, after one prepares one’s spectra for 2D-COS analysis, then the application of the algorithm itself is much less subject to “pilot influence.”
In the example shown here, the goal was to use Raman spectra in combination with 2D-COS analysis to understand how the addition of propyl side groups to the polymer backbone influences the structure of the polymer at the atomic level. We saw that even though assignment of functional group vibrations using classical spectroscopic tools was not robust, the 2D-COS did provide information on where the polymer was changing and what happened first.
Acknowledgment
The samples for this manuscript were kindly supplied by Professor Isao Noda at the University of Delaware and his col- league, Dr. Reva Street, who is now on the faculty at Drexel University.
References
(1) I. Noda, S.B. Lindsey and D. Caraway, in Plastics from Bacteria: Natural Functions and Applications, Microbiology Monographs, Volume 14, G.Q. Chem, Editor (Springer Verlag, Berlin, Germany, 2010), pp. 237–255.

Fran Adar is the Principal Raman Applications Scientist for Horiba Scientific in Edison, New Jersey. Direct correspondence to: SpectroscopyEdit@ mmhgroup.com. ●

New Raman Spectroscopy Method Enhances Real-Time Monitoring Across Fermentation Processes
April 15th 2025Researchers at Delft University of Technology have developed a novel method using single compound spectra to enhance the transferability and accuracy of Raman spectroscopy models for real-time fermentation monitoring.
Nanometer-Scale Studies Using Tip Enhanced Raman Spectroscopy
February 8th 2013Volker Deckert, the winner of the 2013 Charles Mann Award, is advancing the use of tip enhanced Raman spectroscopy (TERS) to push the lateral resolution of vibrational spectroscopy well below the Abbe limit, to achieve single-molecule sensitivity. Because the tip can be moved with sub-nanometer precision, structural information with unmatched spatial resolution can be achieved without the need of specific labels.
AI Shakes Up Spectroscopy as New Tools Reveal the Secret Life of Molecules
April 14th 2025A leading-edge review led by researchers at Oak Ridge National Laboratory and MIT explores how artificial intelligence is revolutionizing the study of molecular vibrations and phonon dynamics. From infrared and Raman spectroscopy to neutron and X-ray scattering, AI is transforming how scientists interpret vibrational spectra and predict material behaviors.