The Benzene Fingers, Part II: Let Your Fingers Do the Walking Through the Benzene Fingers
Spectroscopy
With the theoretical background of benzene analysis laid out in part 1 of this series, we now know what fundamental, overtone, and combination bands look like. Here, I show that the benzene fingers are a series of overtone and combination bands that can be used to distinguish substituted benzene rings from each other when other methods do not work. I review the benzene finger patterns for mono-, ortho-, meta-, and para- substituted benzene rings, and describe an easy mnemonic in which you use your fingers to help you remember the patterns.
With the theoretical background of benzene analysis laid out in part I of this series, we now know what fundamental, overtone, and combination bands look like. Here, we show that the benzene fingers are a series of overtone and combination bands that can be used to distinguish substituted benzene rings from each other when other methods do not work. We also review the benzene finger patterns for mono-, ortho-, meta-, and para-substituted benzene rings, and describe an easy mnemonic in which you use your fingers to help you remember the patterns.
In an earlier installment (1), I introduced the chemical structures and nomenclature for mono- and disubstituted benzene rings, and showed how the presence or absence of the ring bend at 690 ± 10 cm-1 and the position of the out-of-plane aryl C-H wag together can be used to distinguish mono-, ortho-, meta-, and para-isomers from each other. These peak positions are summarized in Table I.
However, we discovered that under certain circumstances Table I is not sufficient to distinguish the four structures in question from each other. For example, both mono- and meta-isomers have the ring bend present and have C-H wagging peaks between 770 and 750 cm-1. What then are we to do?
In part I of this series (2), we discovered that there is a series of overtone and combination bands between 2000 and 1650 cm-1 that I call the benzene fingers, that are another tool to distinguish substituted benzene rings from each other. I mentioned that the benzene fingers are a series of overtone and combination bands, and then spent the rest of the column explaining what those are.
In this final installment of the benzene fingers saga, I show the actual peak patterns for mono-, ortho-, meta-, and para-rings, and share with you a silly but effective mnemonic using your fingers to help you remember the benzene ring patterns. Hence the title of this installment.
The Benzene Finger Patterns for Mono- and Disubstituted Benzene Rings
The benzene fingers for mono- and disubstituted benzene rings are not defined by peak positions but by the number, spacing, and relative intensity of a series of peaks between 2000 and 1650 cm-1. As I have stated previously, infrared (IR) spectral interpretation is not about memorizing peak positions but rather it is an exercise in pattern recognition. The benzene fingers prove this point.
In a previous column (1), we looked at the spectra of toluene and the three xylenes. If you go back to that column installment, and use a magnifying glass, you might be able to see the benzene finger patterns. These patterns have been expanded and plotted in absorbance for you in Figure 1.
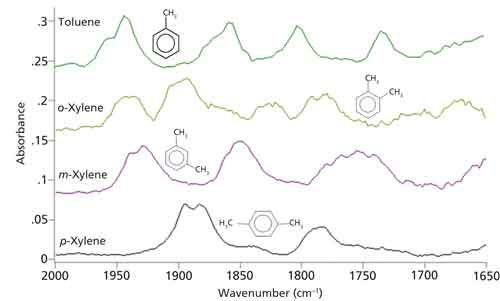
Figure 1: The benzene finger patterns for mono-, ortho-, meta-, and para-substituted benzene rings.
The pattern for mono-substituted rings is four well separated peaks, and for ortho-substituted rings it is four peaks close together. For meta-substituted rings there are three peaks, while for para-substituted rings there are two peaks. Mono-, ortho-, meta-, para- . . . four peaks, four peaks, three peaks, two peaks . . . four, four, three, two . . . whose patterns can be remembered using your fingers as shown in Figures 2a–2d.
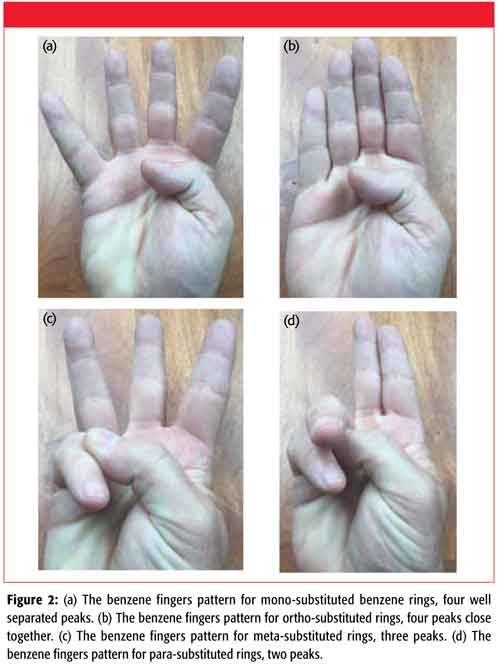
Figure 2: (a) The benzene fingers pattern for mono-substituted benzene rings, four well separated peaks. (b) The benzene fingers pattern for ortho-substituted rings, four peaks close together. (c) The benzene fingers pattern for meta-substituted rings, three peaks. (d) The benzene fingers pattern for para-substituted rings, two peaks.
When I was a boy I was a member of the Cub Scouts, and when I got older the Boy Scouts. The Cub Scout salute used two fingers and the Boy Scout salute used three fingers. Another way I remember the benzene fingers for mono- and disubstituted benzene rings is four fingers, four fingers, Boy Scout salute, Cub Scout salute. This messing around with benzene and human fingers may seem silly, but I guarantee after reading this column you will never forget the benzene ring patterns for mono-, ortho-, meta-, and para-substituted benzene rings.
I stated above that Table I sometimes can’t be used to distinguish between mono- and meta-isomers. The benzene fingers solve this problem-mono-substituted benzene has four benzene finger peaks and meta-substituted benzene has three benzene finger peaks–problem solved. In general, when I need to determine the substitution pattern around a benzene ring I use the peak positions in Table I first; if they don’t work for whatever reason I will use the benzene fingers. If Table I can be used to determine a substitution pattern, I still consult the benzene fingers as a backup to my initial conclusion.
The benzene fingers are easy to use but suffer from some problems. First of all they are inherently weak because they are a series of overtone and combination bands (2). Even in pure form these peaks can be 10 times or more smaller than the fundamental bands of a molecule. Thus, at a short pathlength, or in a mixture where the benzene ring is in low concentration, these peaks may be not big enough to see. A second problem with the benzene fingers is that they show up in a busy region of the spectrum where lots of large peaks can appear and mask their presence. Carbonyl (C=O) stretching peaks frequently fall between 1900 and 1600 cm-1, are often intense, and have been known to mask the presence of the benzene fingers (3). Obviously, if you can’t see the benzene fingers you can’t use them to help determine benzene ring substitution patterns.
Other Types of Aromatic Rings
When I first introduced you to benzene rings (4) we talked about aromatic rings in general and what makes them structurally and spectroscopically unique. Since the benzene molecule contains six hydrogens, it can have up to six substituents. As discussed previously (2), the reason I limited the discussion to mono- and disubstituted benzene rings is that I believe they are the most common aromatic rings, and have the easiest to interpret spectra. The spectra of benzene rings with three, four, or five substituents display C-H stretches above 3000 cm-1 and ring modes like their less substituted cousins. Similarly, the presence or absence of a ring bend and the position of one or more C-H wags can be used to determine their substitution pattern. Highly substituted rings also exhibit their own unique benzene finger patterns.
Benzene rings can also be found in structures where they are fused, with adjacent rings sharing two carbons. These are called polycyclic aromatic hydrocarbons (PAHs) (5). Some examples of simple PAHs are seen in Figure 3.
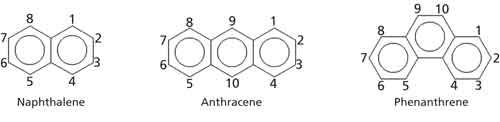
Figure 3: Some examples of polycyclic aromatic hydrocarbons. The numerals represent the carbon numbering scheme for each specific ring system.
Naphthalene is the simplest PAH containing two fused benzene rings. It is the smelly ingredient commonly found in moth balls. There are two isomers possible for three benzene rings fused together, anthracene and phenanthrene, as seen in Figure 3. Because PAHs have more atoms than benzene rings, their spectra are more complicated. However, they exhibit many of the same features already discussed for benzene rings including C-H stretches above 3000 cm-1, aryl C-H wags, and ring bends. As mentioned above, some of these features can be used to distinguish isomers from each other. For example, the two isomers of a mono-substituted naphthalene can be distinguished from each other by their IR spectra.
A heterocyclic aromatic hydrocarbon is an aromatic ring that contains a noncarbon or “heteroatom.” Examples of heteroatoms include nitrogen and oxygen. Pyridine and substituted pyridines are heterocyclic aromatic hydrocarbons. Some example structures of these are seen in Figure 4.
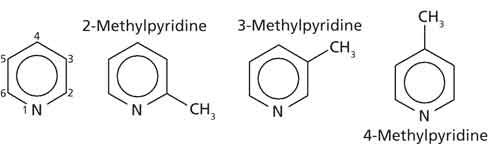
Figure 4: Pyridine and the family of mono-substituted pyridines, examples of heterocyclic aromatic hydrocarbons.
Like other aromatic rings, heterocyclic aromatic hydrocarbons have C-H stretches above 3000 cm-1, ring modes, and aryl C-H wags. These peaks can be used, for example, to distinguish the three mono-substituted pyridines shown in Figure 4 from each other. A discussion of the IR spectra of highly substituted benzene rings, polycyclic aromatic hydrocarbons, and heterocyclic aromatic hydrocarbons will be the subjects of future columns.
References
- B.C. Smith, Spectroscopy31(5), 36–39 (2016).
- B.C. Smith, Spectroscopy31(7), 30–34 (2016)
- B.C. Smith, Infrared Spectral Interpretation: A Systematic Approach (CRC Press, Boca Raton, Florida, 1999).
- B.C. Smith, Spectroscopy31(3), 34–37 (2016).
- R. Morrison and R. Boyd, Organic Chemistry 7th Edition (Allyn and Bacon, New York, 2003).
Brian C. Smith, PhD, is a Senior Infrared Product Specialist for PerkinElmer, based in San Jose, California. Before joining PerkinElmer, he ran his own FT-IR training and consulting business for more than 20 years, and taught thousands of people around the world how to improve their FT-IR analyses and interpret infrared spectra. Dr. Smith has written three books on infrared spectroscopy: Fundamentals of FTIR and Infrared Spectral Interpretation, both published by CRC Press, and Quantitative Spectroscopy: Theory and Practice published by Academic Press. He has published a number of papers in peer-reviewed journals and is a co-inventor on a patent for an FT-IR method to monitor dust exposure in coal mines.

AI Shakes Up Spectroscopy as New Tools Reveal the Secret Life of Molecules
April 14th 2025A leading-edge review led by researchers at Oak Ridge National Laboratory and MIT explores how artificial intelligence is revolutionizing the study of molecular vibrations and phonon dynamics. From infrared and Raman spectroscopy to neutron and X-ray scattering, AI is transforming how scientists interpret vibrational spectra and predict material behaviors.
Real-Time Battery Health Tracking Using Fiber-Optic Sensors
April 9th 2025A new study by researchers from Palo Alto Research Center (PARC, a Xerox Company) and LG Chem Power presents a novel method for real-time battery monitoring using embedded fiber-optic sensors. This approach enhances state-of-charge (SOC) and state-of-health (SOH) estimations, potentially improving the efficiency and lifespan of lithium-ion batteries in electric vehicles (xEVs).
New Study Provides Insights into Chiral Smectic Phases
March 31st 2025Researchers from the Institute of Nuclear Physics Polish Academy of Sciences have unveiled new insights into the molecular arrangement of the 7HH6 compound’s smectic phases using X-ray diffraction (XRD) and infrared (IR) spectroscopy.