Determination of Low Levels of Chromium in Biological Samples by ICP-MS Using Hydrogen as a Reaction Gas
Spectroscopy
Chromium is a key ingredient in a number of metal alloys used for metal implants which, despite being coated with inert surface layer coatings, can break down over time as a result of biocorrosion. Leeching of metal ions from implants into surrounding bone, tissue, and body fluids can cause severe health problems. Although the toxicity of the metal is low, there is a potential health risk if chromium ions enter the body. In ICP-MS, the chromium (52Cr) signal can be affected by interference from the recombination of background plasma 40Ar and sample-specific matrix 12C. To eliminate this interference, hydrogen can be used in the iCRC as a reaction gas to allow accurate analysis of 52Cr. Results using certified clinical standards of chromium in blood, plasma, urine, and serum clearly demonstrate the benefit of using hydrogen as a collision gas to remove the argon-carbide polyatomic interference. This study shows that ICP-MS is an essential tool for clinical monitoring of metal ions in complex matrices and that hydrogen iCRC gas allowed for greater accuracy and a lower level of quantitation in clinical matrices.
Chromium is a key ingredient in a number of metal alloys used for metal implants which, despite being coated with inert surface layer coatings, can break down over time as a result of biocorrosion. Leeching of metal ions from implants into surrounding bone, tissue, and body fluids can cause severe health problems. Although the toxicity of the metal is low, there is a potential health risk if chromium ions enter the body. In inductively coupled plasma–mass spectrometry (ICP-MS), the chromium (52Cr) signal can be affected by interference from the recombination of background plasma 40Ar and sample-specific matrix 12C. To eliminate this interference, hydrogen can be used in the collision–reaction cell as a reaction gas to allow accurate analysis of 52Cr. Results using certified clinical standards of chromium in blood, plasma, urine, and serum clearly demonstrate the benefit of using hydrogen as a collision gas to remove the argon-carbide polyatomic interference. This study shows that ICP-MS is an essential tool for clinical monitoring of metal ions in complex matrices and that hydrogen collision–reaction cell gas allowed for greater accuracy and a lower level of quantitation in clinical matrices.
Inductively coupled plasma-mass spectrometry (ICP-MS) became established as a technique that is an important tool in the detection and quantification of trace and ultratrace elements in human body fluids over 25 years ago (1). As a technique, ICP-MS gained popularity because of its versatility in determination of multielemental profiles in difficult matrices such as blood and plasma (2). One clinical application for ICP-MS is the determination of metal ions present in body fluids following artificial joint or prosthetic implantation.
Metal human body implants are used for a variety of orthopedic and dental applications, such as hip and knee replacements, with the first metal hip prosthesis being implanted in 1956 (3). Over the past 20–30 years the number of patients receiving implants has grown to more than 1 million and the understanding of which metals and metal alloys are most corrosion resistant, mechanically sound as well as exhibiting low toxicity, has advanced greatly (4). Although modern implants are coated with an inert surface layer coating, over time they will suffer from wear and tear, or biocorrosion. Biocorrosion can be caused by a number of factors such as chemical interactions, changes in pH as well as physical interaction between the implant and surrounding muscle or bone. Release of low levels of metal ions into surrounding bone, tissue, and body fluids can result in allergy or severe health problems.
Chromium is a key ingredient in a number of metal alloys, especially in the widely used cobalt-chromium (Co-Cr) alloy, in which Cr constitutes around 30%. Surgical or orthopedic stainless steel SS316L, the only biocompatible stainless steel, also contains 16–18% Cr (3,4). Although its use in prosthetic body parts is widespread and the toxicity of the metal is low, there is a potential health risk if chromium ions enter the body, particularly if they are transported via blood, plasma, or serum since this transport can lead to systemic poisoning at higher concentrations, which can be signified by their presence in urine (5).
Use of Hydrogen to Reduce Interfering Masses in ICP-MS
Spectroscopic interferences are caused by atomic or molecular ions that have the same mass-to-charge ratio as target analytes. Polyatomic ions commonly originate from a variety of sources including sample matrix, sample preparation reagents, plasma gases, and ambient gases. Common interferences in ICP-MS analysis can be seen in a number of elements such as iron (56Fe), selenium (78Se), calcium (40Ca), arsenic (75As), and chromium (52Cr) as a result of interference with polyatomic and isobaric species formed between argon (Ar), plasma ions (O, N, H), and other matrix ions (Cl, Na, Ca) (6) (see Table I).
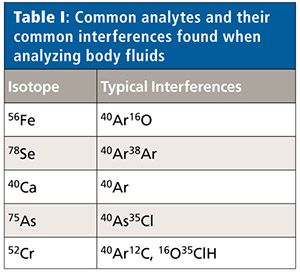
Helium is often used in the collision cell of an ICP-MS system because it will effectively eliminate a wide range of polyatomic interferences via kinetic energy discrimination. However, when argon-based polyatomic interferences occur, helium will not always eliminate interfering ions completely, which results in higher levels of background signal. Alternatively, hydrogen can be used to react with polyatomic species to remove them effectively and allow an accurate quantification of target ions.
Chromium (52Cr) signal can be affected by the recombination from background plasma from 40Ar and sample-specific matrix presence of 12C, which can react to form polyatomic species with the same mass as the target isotope (5). Biological samples such as blood, plasma, and urine assessed in this study have a very high matrix level of carbon, which in combination with argon can have a severe impact on quantification of true chromium levels. To overcome this issue, hydrogen used as a reaction gas can effectively eliminate the interferences to allow more accurate analysis of 52Cr.
Hydrogen is a potentially explosive gas if its concentration in an atmosphere reaches its lower explosion limit (LEL) of 4.1%. An average laboratory would typically require a volume of 2000–5000 L of hydrogen to reach the LEL. To reduce the risk in the laboratory environment of the hydrogen reaction gas in this study, a gas generator was used. These devices produce hydrogen gas on-demand at a low pressure and have low-volume storage, while still providing the required purity and volume of reaction gas for the ICP-MS system. The hydrogen generator produces hydrogen through the electrolysis of deionized water via a proton exchange membrane (PEM) cell.
Experimental
All analyses were carried out using an Analytik Jena PlasmaQuant MS ICP-MS system with a Peak Scientific Precision Hydrogen 300cc generator for supply of hydrogen reaction gas.
Samples
Certified reference materials were used to determine accuracy and precision of detected amounts of chromium in blood, plasma, and urine matrices (ClinChek Whole Blood level I, II, and III, Plasma level I and II, and Urine level I and II, Recipe, Germany).
The diluent solution contained 2% ammonia, 0.1% EDTA, 0.01% Triton-X, 1% isopropanol, and 200 ppb gold. Urine and serum samples were diluted 1:10. Blood samples were diluted 1:20.
Calibration
A five-level calibration curve was established that ranged from 0.1 to 10 µg/L (Figure 1). Instrument operating conditions are shown in Table II.
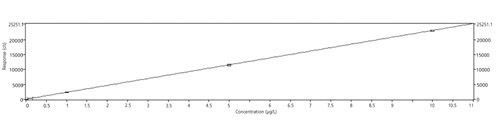
Figure 1:52Cr calibration curve results.
Results
Calibration
Results from the calibration show that the use of hydrogen as the collision gas reduced the background to an extremely low level in the blank, with just 55 c/s detected (Figure 1). This level of background allows highly accurate analysis down to very low levels compared with calibration samples run using helium collision gas, where the blank reading for 52Cr was 955 c/s (data not shown). Linearity of calibration standards was very good with a correlation coefficient R2 = 0.999999. The calibration results show levels of chromium can be detected at 0.1 ppt, with scope to increase sensitivity below this level when using hydrogen collision gas, whereas use of helium for 52Cr detection resulted in reduced sensitivity by one order of magnitude, because of increased background noise.
Measured 52Cr Concentration Versus Reference Materials
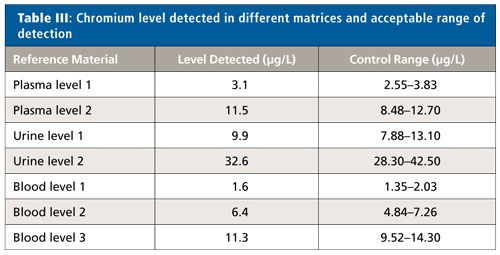
It is essential that measured concentrations fit within the specified clinical reportable range (CRR) so that quantitative results can be reported, even after dilution, concentration, or any other pretreatment used to extend the analytical measurement range (AMR) of samples. The measured concentrations of chromium in the different reference materials were in perfect agreement with the certified control range, showing the accuracy of the method employed in this study (Table III). Therefore quantitative measurement of 52Cr using this method would be valid for clinical sample measurement.

Summary
Over the past 25 years, ICP-MS has emerged as the leading technique for monitoring metal ions in complex matrices, particularly in clinical applications. Biocorrosion is a process that can cause release of metal ions from an implant into surrounding tissue, which can ultimately migrate to distant organs and can be detected in body fluids. The effect of ions leaching into peri-implant tissue can include metal allergy, granuloma formation, and occasionally carcinoma. It is therefore essential to be able to reliably detect metal ion levels in body fluids of patients with metal body implants to enable timely intervention in the case of implants having undergone substantial biocorrosion.
As a key constituent of a number of alloys used in metal body implants, 52Cr ions could potentially cause health effects when released from implants via biocorrosion. Polyatomic interference from matrix-specific 12C reacting with 40Ar makes accurate trace-level detection of 52Cr difficult when using helium in the collision cell, since it does not effectively eliminate the interfering ions. Hydrogen, however, offers an improved mechanism of elimination of interference through reaction with polyatomic species. This study showed that when using hydrogen as the collision-reaction gas, negligible background levels of polyatomic 12C4Ar are present, meaning lower detection limits were possible.
Body fluids as matrices offer different challenges in terms of potential interference and this study has demonstrated that monitoring of 52Cr in blood, plasma, and urine is possible when using hydrogen as the collision-reaction gas, with a high level of linearity achievable in all three matrices. Results of 52Cr in reference samples was within the manufacturer’s specified error range, meaning that regardless of matrix, 52Cr could be reliably detected to parts-per-trillion levels.
References
- T.D.B. Lyon, G.S. Fell, R.C. Hutton, and A.N. Eaton, J. Anal. Atom. Spec.3, 265–271 (1988).
- M-A. Vaughan, A.D. Baines, and D.M. Templeton, Clin. Chem.37(2), 210–215 (1991).
- H. Matusiewicz, Acta Biomater. 10, 2379–2403 (2014).
- D. Cadosch, E. Chan, O.P. Gautschi, and L. Filgueira, J. Biomed. Mater. Res. Part A91A(4) 1252–1262 (2009).
- J.P. Goullé, E. Saussereau, J. Grosjean, C. Doche, L. Mahieu, J.M. Thouret, M. Guerbet, and C. Lacroix, Forensic Sci. Int.217(1–3), e8-e12 (2012).
- T.W. May and R.H. Wiedmeyer, Atom. Spec.19(5), 150–155 (1998).
Ed Connor is an application specialist with Peak Scientific Instruments in the UK. Andrew Ryan is an ICP-MS product manager with Analytik Jena AG in Germany. Peio Riss is an ICP-MS Application Specialist with Analytik Jena AG in France. Direct correspondence to:

Smarter Sensors, Cleaner Earth Using AI and IoT for Pollution Monitoring
April 22nd 2025A global research team has detailed how smart sensors, artificial intelligence (AI), machine learning, and Internet of Things (IoT) technologies are transforming the detection and management of environmental pollutants. Their comprehensive review highlights how spectroscopy and sensor networks are now key tools in real-time pollution tracking.
New AI Strategy for Mycotoxin Detection in Cereal Grains
April 21st 2025Researchers from Jiangsu University and Zhejiang University of Water Resources and Electric Power have developed a transfer learning approach that significantly enhances the accuracy and adaptability of NIR spectroscopy models for detecting mycotoxins in cereals.
Karl Norris: A Pioneer in Optical Measurements and Near-Infrared Spectroscopy, Part II
April 21st 2025In this two-part "Icons of Spectroscopy" column, executive editor Jerome Workman Jr. details how Karl H. Norris has impacted the analysis of food, agricultural products, and pharmaceuticals over six decades. His pioneering work in optical analysis methods including his development and refinement of near-infrared spectroscopy, has transformed analysis technology. In this Part II article of a two-part series, we summarize Norris’ foundational publications in NIR, his patents, achievements, and legacy.