Fire and Flames
Columnist David Ball discusses the fact that spectroscopy is based upon light and considers the spectroscopy of fire and flame.

Spectroscopy ultimately is based upon light. Historically, the first source of artificial light was probably fire and the flame that accompanied it. Here, let us consider the spectroscopy of fire and flame.

David W. Ball
The impact of fire on human development cannot be underestimated. Fire provided light and heat to ward off cold, and lent itself as a mechanism to cook food so that it could be eaten more easily. It could be used as a tool to help shape metals, decompose ores, and boil water for steam that could be used to power turbines for electricity or cylinders for locomotion. Fire can be devastating when uncontrolled, as in a forest or house fire, or emotionally satisfying, as in a nice fire in the fireplace on a cold winter's night (my own personal favorite; Figure 1).

Figure 1: A fire in a fireplace can be a cozy companion on a cold winter evening. This picture, however, only demonstrates two of three aspects of a fire: the presence of a flame and the emission of light. Fire is also accompanied by the emission of heat.
What is fire? What is flame? How do they apply to spectroscopy? To answer the last question first, there are some simple and complex spectroscopic methods in which a flame has a central role.
Fire
Fire is a rapid oxidation–reduction (or, paradoxically, "redox") reaction that is characterized by the presence of a flame and the emission of light and heat. Fire requires the presence of a fuel, which is oxidized, and an oxidizer, which is reduced. When a simple match burns, the wood or heavy paper of the match is the fuel (along with the chemicals on the head of the match, which initiate the combustion process), while the oxygen in the air is the oxidizer. If one were to try to make a fire on the surface of the Saturnine moon Titan, one would issue a stream of oxidizer such as oxygen into the atmosphere and burn it with the 1% or so of hydrocarbons in Titan's atmosphere (as discussed in Arthur C. Clarke's science fiction novel Imperial Earth). When sodium metal and chlorine gas deflagrate (which is a sort of subsonic combustion), sodium is the fuel and chlorine is the oxidizer; the product is the salt sodium chloride.
Fuels and oxidizers can be premixed, or a fire can depend upon diffusion of one or the other component. For example, a laboratory Bunsen burner has an opening in the bottom to allow for entry of air, which will mix with the fuel before reaching the fire at the top of the burner. If this opening is closed, the fire must depend upon diffusion of oxygen from the surrounding air to sustain combustion. The characteristics of the fires are very different, as shown in Figure 2.

Figure 2: Bunsen burner flames with different amounts of air premixed with the fuel. As the flames progress from 1 to 4, more and more air is premixed with the fuel. Note that not only the color, but also the construction of the flame varies.
There are three parts to fire. Light and heat are the two easy ones to consider. Light is, of course, a transfer of energy caused by an electromagnetic disturbance and has wave and particle properties. Two recent columns (1,2) have discussed its history and its properties. Heat is also a transfer of energy, in this case a transfer of energy due to the differing kinetic energies of adjacent matter. For example, when you hold an ice cube in your hand, it feels cold, as energy is transferred from your hand to the ice cube, warming and eventually melting the ice. The transfer of energy as heat is how we cook food, either directly (by roasting meat) or indirectly (by boiling water which is then used to cook vegetables, for example).
The Flame
The third component of fire is the flame. A flame is the region of the fire in which the chemical processes occurring are so exothermic (heat-emitting) that light is given off. Most of the chemical reactions that occur as part of the combustion process are found in the flame. One can think of a flame as the zone in which the chemical reactions of combustion are occurring.
The color of the flame is due to the chemical species created in the combustion process. These molecules are excited electronically by the thermal energy of the combustion reaction and emit light as they de-excite to a lower electronic state. Many of these molecules are not stable molecules that you might find in a chemistry laboratory; rather, they are short-lived intermediates such as C2, CH, CN, OH, and other species depending upon the fuel and the oxidizer. In fact, spectroscopy has been very useful in determining the exact species that exist in the course of forming a flame.
According to Fristrom and Westenberg (3), the detailed structure of a flame depends upon a host of conditions, among them the identities of the fuel and oxidizer, the amount of premixing or diffusion, the fuel–oxidizer velocity, and the burner shape. A typical methane–oxygen flame can be used as an example. Figure 3 shows a diagram of the zones of a flame. In the transport zone, fuel and oxidizer premix or diffuse. Molecular and thermal transport are the primary processes in this region, and the region is nonluminous. In the very small primary reaction zone, the main initial chemical reactions are taking place, and this region is usually most luminous. Most of the fuel is used up in this region, although the ultimate chemical products of the combustion usually are not present in the primary reaction zone. Following the primary reaction zone is the secondary reaction zone, in which the final products are formed and most of the oxidizer is depleted.
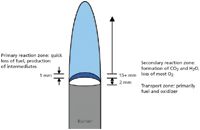
Figure 3: Spatial separation of zones in a typical methaneâoxygen flame. Adapted from reference 3, Figure XIII-3, page 299. Compare this general diagram with the rightmost flame in Figure 2.
The temperature profile of a flame also depends strongly upon the conditions of the flame. However, in most cases, the hottest part of the flame is coincident with the primary reaction zone, and the flame is cooler (but still stays hot enough to burn!) in the secondary reaction zone.
Applications in Spectroscopy
Fire and flames have a few direct applications in spectroscopy. A method that uses a flame directly is the flame test, a simple, qualitative test for the presence of a particular element that is commonly taught in high school and freshman university laboratories. Different ions of different elements (usually metals) make a flame burn with a characteristic color when introduced into the reaction zone; the different colors are caused by the different quantized energy levels of the electrons in the different atoms. Figure 4 shows examples of sodium and copper. The problem with this particular test is that different elements can show very similar colors, and more sophisticated spectral analysis is necessary to identify the element unambiguously (see below for a useful technique). For example, the three elements barium, antimony, and lead all give a pale green flame test. It might be difficult to differentiate between them by eye unless good standards are available. Similarly, both manganese and molybdenum give a greenish-yellow light when exposed to a flame. Calcium and strontium both give red flames, with strontium causing a more intense red color. Still, in many cases, a quick test for a suspected element (again, usually a metal) can be the flame test.

Figure 4: Results of two qualitative flame tests. Left: a flame indicating the presence of sodium. Right: a flame indicating the presence of copper.
(An interesting application of flame tests is fireworks. Fireworks are colored by the addition of certain metal salts into the pyrotechnic mixtures; the specific metal salt dictates the color of the explosion. Copper makes blue and green, barium makes green, strontium makes red, sodium makes yellow, antimony makes white, and aluminum makes silver [4].)
There is a form of spectroscopy that uses flames as an integral part of its measurement. In atomic absorption (AA) spectroscopy, a dissolved solute is nebulized and atomized in a wide, narrow flame (Figure 5). Light generated from a lamp composed of the element being analyzed is passed through the flame. The atomized element in the flame absorbs the lamp emission, allowing the observer to identify and quantify the amount of element in the sample. Typically, hollow cathode lamps are used. One advantage is that the light emitted by a hollow cathode lamp consists of lines that have a very narrow width, a width that can be narrower than the absorption profile of the sample. Unfortunately, the sample in the flame can emit some characteristic light also. This potential problem is solved by using a modulator, such as a chopper, in the light path before the flame. A detector will see a constant signal from the flame emission and a variable signal from the modulated source. A lock-in amplifier isolates the variable signal and amplifies it.
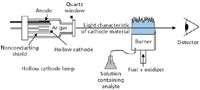
Figure 5: General schematic of an atomic absorption spectrometer.
The cathodes of the source can be constructed of more than one element, which allows for simultaneous analysis of more than one element in a sample.
Careful calibration must be done in AA spectroscopy if it is to be used for quantitative purposes. Lamp voltages and currents, emission and absorption profiles, and instrumental factors can introduce deviations from Beer's law (5). When properly calibrated, AA spectroscopy has detection limits in the sub-part-per-million range, with only a small sample size required.
Conclusion
Fire — perhaps mankind's first tool. It is fitting, therefore, that fires and flames are an important tool in the spectroscopist's arsenal.
David W. Ball is a professor of chemistry at Cleveland State University in Ohio. Many of his "Baseline" columns have been reprinted in book form by SPIE Press as The Basics of Spectroscopy, available through the SPIE Web Bookstore at www.spie.org. His most recent book, Field Guide to Spectroscopy (published in May 2006), is available from SPIE Press. He can be reached at d.ball@csuohio.edu his website is academic.csuohio.edu/ball.
References
(1) D.W. Ball, Spectroscopy 21(6), 30–33 (2006).
(2) D.W. Ball, Spectroscopy 22(3), 14–20 (2007).
(3) R.M. Fristrom and A.A. Westenberg, Flame Structure (McGraw-Hill, New York, 1965).
(4) R. Lancaster, Fireworks: Principles and Practice (Chemical Publishing Co., New York, 1992).
(5) D.W. Ball, Field Guide to Spectroscopy (SPIE Press, Bellingham, Washington, 2006).

LIBS Illuminates the Hidden Health Risks of Indoor Welding and Soldering
April 23rd 2025A new dual-spectroscopy approach reveals real-time pollution threats in indoor workspaces. Chinese researchers have pioneered the use of laser-induced breakdown spectroscopy (LIBS) and aerosol mass spectrometry to uncover and monitor harmful heavy metal and dust emissions from soldering and welding in real-time. These complementary tools offer a fast, accurate means to evaluate air quality threats in industrial and indoor environments—where people spend most of their time.
NIR Spectroscopy Explored as Sustainable Approach to Detecting Bovine Mastitis
April 23rd 2025A new study published in Applied Food Research demonstrates that near-infrared spectroscopy (NIRS) can effectively detect subclinical bovine mastitis in milk, offering a fast, non-invasive method to guide targeted antibiotic treatment and support sustainable dairy practices.