Geochemical Analysis Using Laser-Induced Breakdown Spectroscopy
Laser-induced breakdown spectroscopy (LIBS) is an ideal method for elemental analysis of geological samples. Although X-ray fluorescence (XRF) can measure a wide variety of elements, it struggles to measure elements lighter than silicon, meaning that many key crustal elements cannot be measured. In addition, as a fast, standoff method, LIBS can be employed in a variety of geometries with various distances from the target. LIBS has famously been employed by the National Aeronautics and Space Administration (NASA) on their Mars rovers, first in the ChemCam instrument and now in the SuperCam instrument. This article summarizes both the basic methodology and the most successful calibration and quantification methods to date.
Geomaterial analysis is a constantly advancing field. As both imaging and spectroscopic methods improve, analysts are better able to pinpoint valuable deposits and rapidly grade and sort ore samples for resource extraction. The improved efficiency of both imaging and spectroscopic methods benefits all types of resource exploration, from hard rock mining to trace rare earth discovery to oil and gas exploration. Better analysis tools lessen waste, lower energy and water usage, and ensure the high quality of the final product.
Traditionally, extractive sampling methods have been used for geochemical analysis. For individual rock samples, precise microtome methods evolved to subsample small, indexed portions of the rock sample for inductively coupled plasma–mass spectrometry (ICP-MS) analysis. Similarly, for bulk analysis, subsampling methods have been determined by several organizations, one being the American Society for Testing and Materials (ASTM), to sample rock samples as large as railroad cars for ICP-MS analysis. Detection limits with ICP-MS are element- and method-dependent but can easily be in the parts per billion (ppb) range. Geological laboratories located at or near mine sites can typically turn around samples as fast as 6–8 h. Other techniques that use simpler instruments, such as atomic absorption spectroscopy (AAS), are commonly used for particular elements.
X-ray fluorescence (XRF) is popular for real-time testing without extractive sampling. XRF is available in various formats, such as portable XRF “guns,” cross-belt configurations, and custom configurations such as the Bruker S2 Kodiak. Because X-rays penetrate materials to specific material-dependent depth, XRF accomplishes near-surface bulk analysis conveniently. There are some matrix effects that occur while using XRF, meaning that an universal calibration is not error-free. These effects arise primarily from emitted X-rays being absorbed as they exit the sample, or exciting further lower-energy fluorescence. Furthermore, XRF needs to be accomplished at a very close range to the sample, ideally touching the sample, for efficient collection of X-rays. XRF is effective for elemental analysis of elements greater than about 14 (silicon) on the periodic table. A lack of absorption of X-rays severely limits accuracy for lighter elements.
Prompt gamma neutron activation analysis (PGNAA) is also used for cross-belt analysis. Neutrons from a radioactive source (typically Californium-252) interact with the nucleus of elements present in the sample, causing the emission of gamma rays onto a suitable detector. Thermo Fisher and Scantech are the leading suppliers of PGNAA instruments. PGNAA is particularly popular in the cement industry. However, the relatively large size and radiation protection requirements render it a limited technique.
For traditional thin-section analysis, laser ablation ICP-MS (LA-ICP-MS) has become the standard. A femtosecond laser, short wavelength excimer laser, or wavelength-converted Nd:YAG laser is used to ablate material in micrometer-sized spots from the sample. The ablated material is transported to an ICP-MS instrument for sensitive elemental analysis. When used in conjunction with automated systems from suppliers like Teledyne CETAC, Elemental Scientific Lasers, and Applied Spectra, LA-ICP-MS, as a technique, provides the user with detailed micrometer-level analysis of geosamples, including full elemental composition. Moderately-sized samples can take in excess of 12 h to run because of the scanning speeds, and the cost of the entire system usually exceeds $400,000.
LIBS Implementation for Geomaterials
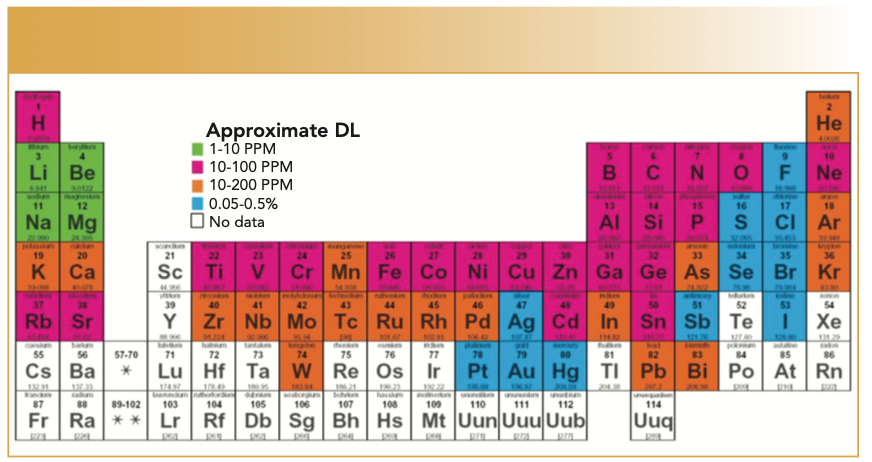
The variety of needs for measuring geomaterials, and the considerations of existing techniques noted above, allows ample room for using laser-induced breakdown spectroscopy (LIBS) for geomaterial analysis. LIBS forms a plasma directly from material ablated from the surface of a sample. LIBS also excites elements across the periodic table, but preferentially excites lighter elements (atomic numbers 1–20) as well as first-row transition elements, which together make up the bulk of crustal materials. Detection limits are typically in the tens of parts per million (ppm) range, and are highly dependent on the matrix and the type of laser used in the application. Figure 1 shows a conservative estimation of detection limits over many of the elements in the periodic table.
FIGURE 1: Approximate (conservative) detection limits obtainable with LIBS, highly dependent on the material and laser(s) and spectrometer used for analysis.
A recent review by Harmon and Senesi in March 2021 identified 640 publications in the scientific literature on geomaterials analysis using LIBS (1). The reader is referred to this excellent source for details on particular configurations; the article focuses primarily on laboratory applications but has some suggestions on how LIBS can be applied outside of the laboratory as well.
Basic implementation choices include the choice of laser source for excitation and the choice of spectrometer for emission detection. For nanosecond-duration laser pulses, shorter-wavelength lasers provide more effective ablation, whereas longer-wavelength lasers couple into the evolving plasma more effectively. For this reason, 193-nm excimer lasers and 213-nm Nd:YAG lasers are preferred among nanosecond-duration lasers for LIBS on glassy materials (including geomaterials) that are partially transparent. These lasers form weaker plasmas, but this is often more than compensated for by the improved material ablation.
The ablation process with a nanosecond laser is largely a thermal process, forming a heat-affected zone (HAZ) in the material. The thermal nature of the process means that the ablation is not stoichiometric, and the range of particulate sizes formed in the ablation and subsequent nucleation process have varying chemistries. The formed particles are then subject to the analytical plasma, which has an effective temperature and electron density that is a function of the laser pulse energy and wavelength. For these reasons the LIBS plasma emission must be calibrated independently for each material with a given system.
The ablation process with a femtosecond laser does not rely on thermal processes. Because the femtosecond laser pulse duration is much shorter than the characteristic vibration (phonon time) in the material, there is no time for heat transfer during the laser pulse. The result is much more stoichiometric ablation, with craters that look quite symmetric and smooth, with no HAZ. The plasma from a femtosecond laser forms following the laser-material interaction and is typically weaker than the plasma from a nanosecond laser, exhibiting lower temperatures and electron densities. Thus, the plasma formed from the femtosecond laser also exhibits higher absolute detection limits. Because of the additional cost of a femtosecond laser, the cons of using a femtosecond laser typically outweigh the advantage of using them for stoichiometric ablation. As a result, femtosecond lasers are usually used only in the laboratory.
Spectrometers are the other key choice of hardware. Many applications can use the miniature crossed Czerny-Turner type spectrometer pioneered by Ocean Optics (now Ocean Insight) in the 1990s. Properly synchronized, spectrometers that cover the range of interest (as broad as 190–800 nm is typical) with at least 0.1 nm resolution are sufficient for most applications. The CCD or CMOS detectors typically have a minimum gate time of roughly 1 millisecond, so the spectrometer acquisition is triggered with a specified delay immediately following the laser pulse and the entire LIBS emission is collected. This results in a non-negligible background, which must be subtracted, and sacrifices some signal-to-noise (S/N).
In most cases, the alternative to banks of miniature spectrometers is an echelle spectrometer mated to an intensified CCD (ICCD) camera or an electron-multiplying CCD (EMCCD) camera, such as those from Andor Technology, Roper Scientific, or Raptor Photonics. Several companies offer echelle spectrometers that are widely used with LIBS, notably Catalina Scientific and LTB Lasertechnik. The echelles produce higher resolution than the miniature spectrometers, and the ICCD and EMCCD detectors are much more sensitive than the nonintensified CCD and CMOS detectors. This combination yields improvement in S/N and thus results in better detection limits. Downsides include a higher price and higher susceptibility to temperature fluctuations and vibrations than the miniature spectrometer solution.
Innovative Applications
One of the fastest-evolving applications of LIBS in geosciences surrounds elemental mapping of samples. A recent review paper summarizes a great deal of relevant literature (2). LIBS has some notable features compared with laser ablation (LA)-ICP-MS, as well as a few drawbacks. Positives are solid detection limits across the periodic table, particularly for several nonmetals and light elements that are not detected as efficiently by LA-ICP-MS. Simultaneous spectral acquisition means that transport-related issues in LA-ICP-MS and parameterization associated with sequential (sometimes known as scanning) mass spectrometers are avoided. High-speed spectral acquisition means that full spectra can be acquired at up to kilohertz (kHz) rates. However, LA-ICP-MS still has superior detection limits for some elements (particularly beyond row four of the periodic table) and for isotopes, which LIBS cannot detect without either very high-resolution spectrometers or molecular emission measurements.
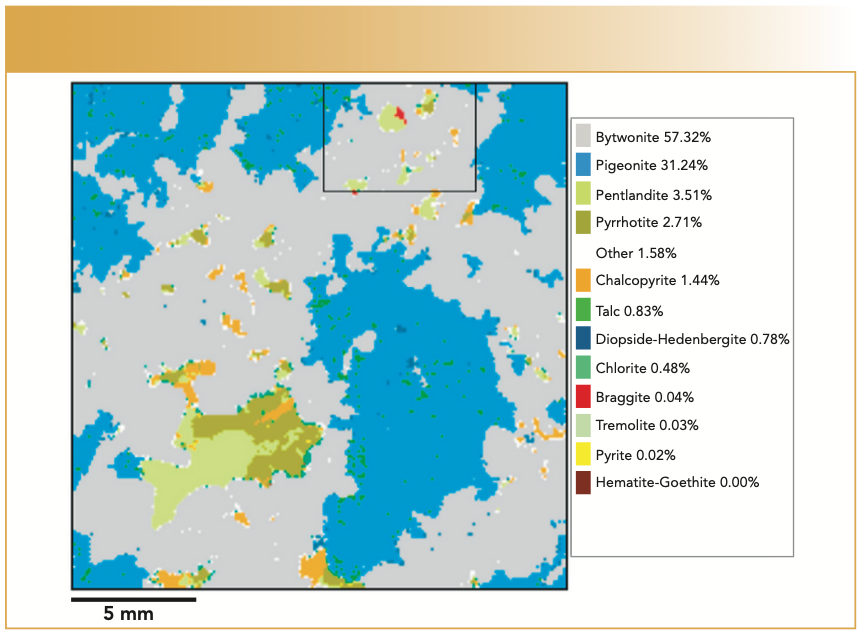
An example of such mapping is included in Figure 2, where the Curiosity LIBS system from Elemission was used to map 20 x 20 mm and larger regions of mineral samples with 50 μm resolution at 1000 Hz (3). The LIBS system was previously calibrated with a machine learning approach on a separate sample, with ground-truth concentration data provided by the Tescan Integrated Mineral Analyzer (TIMA), a scanning electron microscope-based analyzer. Tests showed good agreement on mineral mass contribution between TIMA and the LIBS analyzer after calibration. Of particular interest and indicated in Figure 2 was the identification of braggite, a mineral containing platinum, palladium, and nickel. Lightigo has recently introduced the FireFly LIBS chemical imaging and analysis system, which is equipped for resolution down to 10 μm, depth profiling, and data acquisition rates up to 100 Hz.
FIGURE 2: Mineral map of a 20 x 20 mm sample acquired at 1000 Hz and with 50 μm resolution, from reference (2), shared under the terms and conditions of the Creative Commons Attribution (CC BY 4.0) license.
The ChemCam LIBS instrument has been mentioned in the press for nearly a decade since touching down on the surface of Mars on the Curiosity rover on August 6, 2012. The ChemCam LIBS instrument quantifies elements and minerals up to 7 m from the rover using LIBS. The ChemCam has a custom diode-pumped DIVA laser developed by Thales and three custom Ocean Optics (now Ocean Insight) HR2000 spectrometers. Substantial effort was made to obtain calibration data here on Earth under Mars conditions before and concurrent with the flight, and the raw and calibrated results from every spectrum can be found on the Planetary Data System hosted at Washington University of St. Louis. This trove of data has been a gold mine for researchers testing different calibration methods for LIBS on geological samples.
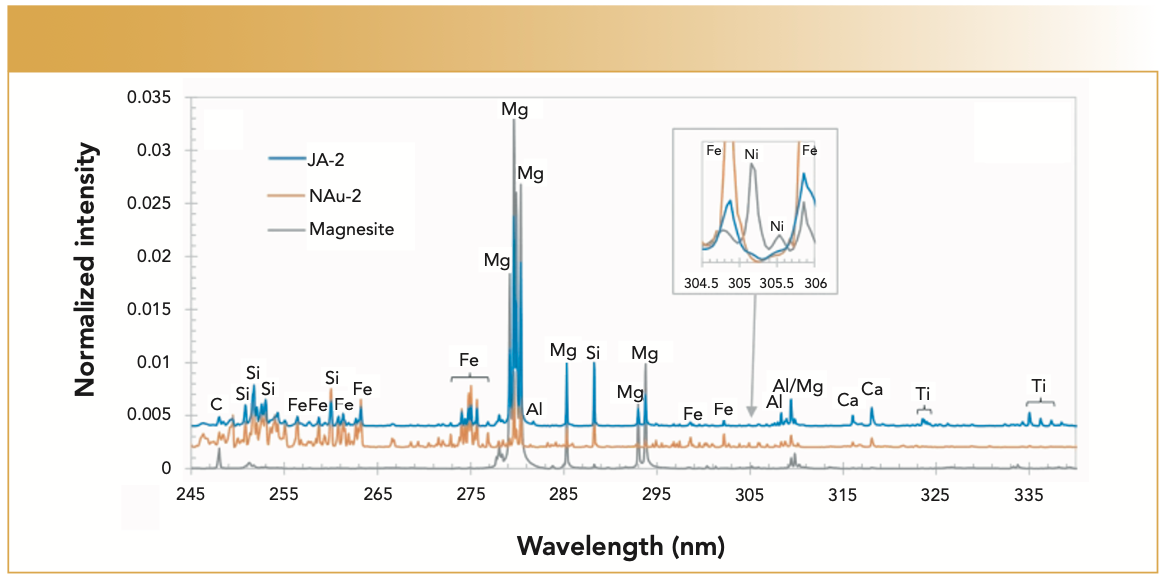
Earlier this year, the next-generation SuperCam touched down with the Perseverance Mars rover. With a similar LIBS system combined with a Raman spectroscopy system, an improved color camera, and an acoustic monitor, SuperCam is providing new data. The design of the SuperCam was covered in the May 2017 issue of Spectroscopy (4). Figure 3 illustrates an example UV LIBS calibration spectrum taken during testing. The resolution on the UV channel is 0.2 full width at half maximum (fwhm) or better (5).
FIGURE 3: Example UV spectrum from the Perseverance Mars rover SuperCam (4), with igneous rock (blue), smectite clay (orange), and magnesium-rich carbonate (gray), from reference (5); insert shows the expanded spectral region. The figure is shared under the terms and conditions of the Creative Commons Attribution (CC BY 4.0) license.
The detailed information available from the LIBS spectra, enabled by the science that has allowed LIBS to be so successful on the Mars rovers, is quickly emerging in field geology and mining applications. SciAps advertises their Z-300 handheld LIBS analyzer for geological applications, and there are many additional handheld LIBS systems to choose from. Having a handheld LIBS instrument with the ability to measure and type geological samples in the field, including light elements, could save geologists from having to bring back pounds of rocks in their backpacks from field expeditions for laboratory analysis.
Perhaps even more exciting are applications on bulk materials for conveyors or drill core analysis. The Ecore from Elemission is one of many commercial examples; LTB Lasertechnik and Ocean Insight are among the companies that provide comprehensive solutions for geological analysis. We have shared before our success in measuring coal elemental composition as well as coal properties (6). In mining and resource exploration, not only can ore composition be assessed, but many other properties can be teased out of LIBS data—examples from the literature include kerogen hydrogen-to-carbon ratios, specific surface area, and rock hardness, among other properties (1). Particular measurements require specialized setups, so whether the sample is in the measurement or in the field, it’s a key step to configure the laser, spectrometer, and sample preparation correctly for success.
The role of analytical instruments in geosciences is a combination of better understanding our natural world and optimizing resource recovery to efficiently and cleanly fuel our economy. It is clear that from the laboratory to the field that LIBS has an increasing role to play in geosciences. We expect that LIBS will “rock on” for some time!
References
(1) R.S. Harmon and G.S. Senesi, J. Appl. Geochem. 128, 104929 (2021).
(2) A. Limbeck, L. Brunnbauer, H. Lohninger, P. Porizka, P. Modlitbova, J. Kaiser, P. Janovszky, A. Keri, and G. Galbacs, Anal. Chim. Acta 1147, 72–98 (2021).
(3) K. Rifai, Minerals 10(10), 918 (2020).
(4) R.C. Wiens, S. Maurice, and F.R. Perez, Spectroscopy 32(5), 50–55 (2017).
(5) R.C. Wiens, et al., Space Sci. Rev. 217(4) (2021). doi: 10.1007/s11214-020-00777-5
(6) S.G. Buckley, Spectroscopy 30(1), 24–31 (2015).
Steven G. Buckley, PhD, is the General Manager of the Applied Systems business at Ocean Insight, an affiliate associate professor at the University of Washington, and has started and advised numerous companies in spectroscopy and in applications of machine learning. He has approximately 40 peer-reviewed publications and 6 patents. His work in practical optical spectroscopy, such as LIBS, Raman, and TDL spectroscopy, dovetails with the coverage in this column, which reviews methods (new and old) in laser-based spectroscopy and optical sensing. Direct correspondence to: SpectroscopyEdit@mmhgroup.com.●


Laser Ablation Molecular Isotopic Spectrometry: A New Dimension of LIBS
July 5th 2012Part of a new podcast series presented in collaboration with the Federation of Analytical Chemistry and Spectroscopy Societies (FACSS), in connection with SciX 2012 — the Great Scientific Exchange, the North American conference (39th Annual) of FACSS.