Polymorph Identification and Analysis Using Ultralow-Frequency Raman Spectroscopy
Results are reported from studies using volume holographic grating filter technology with both visible and NIR excitation wavelengths to analyze multiple forms of the polymorphic active pharmaceutical ingredient carbamazepine.
Ultralow-frequency Raman spectroscopy has commonly been cited as an effective technique for polymorph identification because it accesses the lattice vibrations related to the physical structure of the molecule. However, these frequencies have been difficult and expensive to access with traditional Raman spectrometer systems. Recent advances in volume holographic grating (VHG) filter technology enable rapid acquisition of high-quality ultralow-frequency Raman spectra in the 5–200 cm-1 region using a compact filter system and single-stage spectrograph, greatly simplifying and reducing the cost of utilizing this technique. We report results using this system with both visible and near infrared (NIR) excitation wavelengths to analyze multiple forms of the polymorphic active pharmaceutical ingredient carbamazepine. Both the intense low-frequency Raman bands and fingerprint region transitions are simultaneously captured and compared, demonstrating the range, ease of use, and efficacy of this technique.
In many industries the rapid and reliable detection and identification of polymorphs — solid materials that exist in more than one form or crystal structure — is essential to formulation, analysis, quality assurance, and process control. For example, in pharmaceutical development and manufacturing, active pharmaceutical ingredients (APIs) can have significantly different efficacies and bioavailabilities depending on their form. And for polymers, both the physical properties and chemical interactions will be strongly affected by the molecular and intermolecular structure of the polymeric chain. The consequences of inadequate detection of polymorphs in the manufacturing process can be both costly and time consuming or even hazardous.
Conventional approaches to polymorph discrimination in the fingerprint region often rely on the analysis of relative peak magnitudes, which can be tricky and unreliable. Ultralow-frequency Raman spectroscopy is an effective alternative technique for polymorph identification because it accesses the lattice vibrations related to the physical structure of the molecule or intermolecular interactions (1). However, these frequencies, which reside extremely close to the Rayleigh excitation wavelength, have been both difficult and expensive to access with traditional Raman spectrometer systems. Rayleigh attenuation is critical to all Raman systems, because the process of Raman scattering is extremely inefficient (only about 1 × 10-9 of the incident photons will produce a Raman signal). So, to resolve these extremely weak signals, the excitation wavelength needs to be attenuated with o.d. 8 or better.
Most commercial Raman systems use thin-film edge filters to completely remove the Rayleigh excitation that typically cuts off all signals below about 200 cm-1 from the Rayleigh line, which also blocks the entire anti-Stokes region. The use of most commercially available notch filters will allow capture of anti-Stokes signals, but will also block low-frequency signals because of limitations on their transition bandwidth. When detection of ultralow-frequency signals is required, a multistage (or cascaded) monochromator system has been the historical solution for achieving extremely high Rayleigh reduction while preserving the signals that are close to the laser line, but at the cost of greatly reducing the overall Raman signal as well. These systems are quite large, expensive, and require a great deal of expertise to operate, thus limiting their usefulness in a manufacturing environment.

Figure 1: Spectral range of Raman spectroscopy components indicating both fingerprint and low frequency regions.
Recent advances in volume holographic grating (VHG) filter technology (2,3) have enabled the manufacture of exceptionally narrow bandwidth notch filters with very high throughput. This has led to systems that are capable of rapid acquisition of high-quality, ultralow frequency Raman spectra in the 5–200 cm-1 region (Figure 1). These systems are based on a stable wavelength laser source, a compact series of VHG filters, and a single-stage spectrograph. Each VHG filter has a notch profile that is designed to diffract only one specific wavelength matching the laser and to transmit all other wavelengths. The ultranarrow transition bandwidth of these filters enables extremely high attenuation of the laser wavelength (> o.d. 4), while maintaining very high transmission of nearby Raman signals beyond ~5 cm-1 (Figure 2). This combination of strong Rayleigh attenuation and high broadband transmission enables the system to simultaneously capture both the intense low-frequency Raman bands and fingerprint region transitions, greatly simplifying the overall system and reducing the cost, while improving the sensitivity and reliability of using Raman for polymorph identification and other applications.
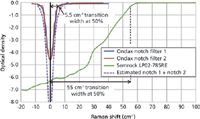
Figure 2: Bandwidth of traditional edge filters vs. VHG notch filters.
Experimental
Experimental measurements were taken with a custom confocal Raman system (Figure 3), comprising a single-mode 785-nm stabilized diode laser (SureLock LM series, Ondax, Inc.), and a series of ultranarrowband VHG filters that were spectrally matched to the laser output wavelength.
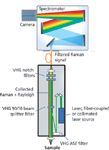
Figure 3: System schematic of the low-frequency Raman spectrometer platform.
First, two VHG amplified spontaneous emission (ASE) suppression filters (NoiseBlock, Ondax, Inc.) were used to remove any nearby ASE from the laser that tends to be the same order of magnitude or larger than the Raman signals and reduce the signal-to-noise ratio (S/N) in the system. Next, a dichroic 90:10 VHG beamsplitter filter (NoiseBlock, Ondax, Inc.) was used to redirect the laser towards the sample. A 10× objective lens focused the laser onto the sample and collected the back-scattered light. The 90:10 beamsplitter then reflected 90% of the Rayleigh scattered light back toward the laser while transmitting the Raman shifted signals. The dichroic nature of the 90:10 beamsplitter resulted in an almost fourfold improvement in collected Raman signal compared to a broadband 50:50 beamsplitter. Finally, two ultranarrowband VHG notch filters (SureBlock, Ondax, Inc.), each having optical density greater than 4.0 were used to further attenuate the collected Rayleigh scattered light while transmitting the Raman signals with an estimated system transmission efficiency of >80%. The entire laser and filter assembly is extremely compact — approximately the size of a ream of notebook paper — and the low power requirements of the laser also make the system operable by battery supply if desired.
It is important to note that the ultranarrow bandwidth of the VHG filters (<0.1 nm) requires the laser to have a very stable wavelength. Nonstabilized diode lasers tend to be highly subject to mode hops, which can shift the laser wavelength outside the blocking range of the filters and result in either reduced Rayleigh suppression or a complete loss of signal in the described configuration.
The filtered signal was focused into a 25-μm core diameter, 0.1-NA step index fiber (HPSC25, ThorLabs), and connected to a high-resolution, high-throughput, single-stage, 0.3-m imaging spectrometer (IsoPlane series, Princeton Instruments). It was equipped with a 1200-line/mm grating and a 1340 × 400 imaging array (Pixis model 400BR with eXcelon, Princeton Instruments) with 20 × 20 μm pixel size and 98% peak quantum efficiency to ensure maximum signal collection and ~1.25 cm-1 resolution; appropriately matched for analysis of the 5–200 cm-1 frequency range.
Results
Two samples having multiple allotropic or polymorphic forms (different structural forms for the same chemical composition) were investigated for this analysis: sulfur and carbamazepine. Sulfur forms more than 30 different allotropes (4), but the most common and easiest to produce are forms α, β, and λ. A sample of α sulfur was placed on a microscope slide and heated with a hot plate while the Raman spectra were measured with the described system as a function of temperature with 80 mW of laser power on the sample and 10 s of total integration time at each temperature setting. The hot plate temperature was monitored with a thermocouple. When the sample temperature was increased above 95.2 °C, the form changed from α to β. Further increasing the temperature above the melting point at 115.21 °C resulted in a second form change to λ. The corresponding Raman spectra for each form were captured and plotted in Figure 4. Note that while there is a corresponding change in magnitude of the peaks in the Raman fingerprint region, there is no obvious shift in the position of the peaks. By comparison, the ultralow-frequency region changes dramatically from one form to another in both magnitude and location of the peaks, enabling clear differentiation of the allotropes.
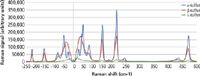
Figure 4: Differentiation of different allotropes of sulfur are easily observed in the low-frequency Raman regime.
Carbamazepine is an anticonvulsant and mood-stabilizing drug that is commonly prescribed in the treatment of epilepsy and bipolar disorder. It has four different polymorphic forms that have been well characterized in the literature (5–9) with form three being the active pharmaceutical ingredient. We obtained pure samples of both form two and form three, as well as the hydrated form and measured the carbamazepine spectra with the same excitation laser and integration conditions (Figure 5). Because the molecules have the same chemical composition, the fingerprint region signals are quite similar, whereas the different structural forms of the polymorphs clearly present themselves as differences in the low-frequency signals. Figure 6 shows additional details of the various polymorphs in the low-frequency regime, including the anti-Stokes signals, which clearly validate the low-frequency measurements. The anti-Stokes signals can often be used to verify low signals in the 5–500 cm-1 region because of the inherent symmetry about the laser line, thus providing additional information that can be used to boost the detection capabilities of the system by removing spurious background noise or signals that break this symmetry.

Figure 5: Complete spectra of carbamazepine, showing clearly differentiating low-frequency signals.
Discussion
Both the sulfur and carbamazepine data demonstrate the value of low frequency Raman signals for the discrimination of different polymorphic forms. The signals found in this region are much stronger in intensity and more clearly differentiating than those in the fingerprint regions (200–2000 cm-1), making them ideal for rapid detection systems and algorithms by reducing the computational complexity required for form discrimination. And because many of the various manufacturing and formulation processes (including temperature, humidity, and pressure) can lead to a change in molecular structure, it is essential that the polymorphic constituents are constantly monitored during formulation to ensure purity of the material. This additional information can be used to inform manufacturing process changes and improve overall yields while reducing manufacturing costs. The compact and affordable nature of the system allows it to be easily integrated into locations where Raman spectroscopy is currently used.
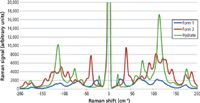
Figure 6: Low frequency and anti-Stokes spectra of carbamazepine.
Summary and Conclusions
We have shown that low-frequency Raman signals can quickly and easily discriminate between different material polymorphs with a compact, VHG-enabled, low-frequency Raman system. The use of ultranarrow-band notch filters in place of conventional edge filters to reduce the Rayleigh signal enables the collection of a wealth of information about the physical structure of the molecule in addition to the chemical composition. Aside from polymorph identification, there are many other applications in which low-frequency Raman signals can be used to gain important new information about materials and boost system sensitivity, such as explosives trace detection and forensics, polymer and industrial chemical development and manufacturing, cancer detection, and basic material science. This new platform will open up the possibility of using low-frequency Raman for these and a multitude of other industrial and scientific uses in the years ahead.
References
(1) A. Hédoux, L. Paccou, Y. Guinet, J.-F. Willart, and M. Descamps, Eur. J. Pharm. Sci. 38, 156–164 (2009).
(2) C. Moser and F. Havermeyer, Appl. Phys. B: Lasers Opt. 95(3), 597–601 (2009).
(3) C. Moser, et al. "Compact Raman spectrometer system for low frequency spectroscopy," Proc. SPIE, 7598, (2010).
(4) R. Steudel and B. Eckert, Top. Curr. Chem. 230, 1–80. doi:10.1007/b12110. ISBN 978-3-540-40191-9 (2003).
(5) H. Pohlmann, C. Gulde, R. Jahn, and S. Pfeifer, Pharmazie 30, 709–711 (1975).
(6) C. Lefebvre, A.M. Guyot-Hermann, M. Draguet-Brughmans, R. Bouche, and J.C. Guyot, Drug Dev. Ind. Pharm. 12, 1913–1927 (1986).
(7) C. Rustichelli, G. Gamberini, V. Ferioli, M.C. Gamberini, R. Ficarra, and S. Tommasini, J. Pharm. Biomed. Anal. 23, 41–54 (2000).
(8) M. Lang, J.W. Kampf, and A.J. Matzger, J. Pharm. Sci. 91, 1186–1190 (2002).
(9) G.M. Day et al., J. Phys. Chem. B 110, 447-456 (2006).
James Carriere and Randy Heyler are with Ondax, Inc., in Monrovia, California. Brian Smith is with Princeton Instruments in Trenton, New Jersey. Direct correspondence to: jcarriere@ondax.com

AI-Powered SERS Spectroscopy Breakthrough Boosts Safety of Medicinal Food Products
April 16th 2025A new deep learning-enhanced spectroscopic platform—SERSome—developed by researchers in China and Finland, identifies medicinal and edible homologs (MEHs) with 98% accuracy. This innovation could revolutionize safety and quality control in the growing MEH market.
New Raman Spectroscopy Method Enhances Real-Time Monitoring Across Fermentation Processes
April 15th 2025Researchers at Delft University of Technology have developed a novel method using single compound spectra to enhance the transferability and accuracy of Raman spectroscopy models for real-time fermentation monitoring.
Nanometer-Scale Studies Using Tip Enhanced Raman Spectroscopy
February 8th 2013Volker Deckert, the winner of the 2013 Charles Mann Award, is advancing the use of tip enhanced Raman spectroscopy (TERS) to push the lateral resolution of vibrational spectroscopy well below the Abbe limit, to achieve single-molecule sensitivity. Because the tip can be moved with sub-nanometer precision, structural information with unmatched spatial resolution can be achieved without the need of specific labels.