Studying the Source of Raw Material and Glaze Formula of Sky Green “Ru-type Ware” and Ru Kuan Ware by EDXRF
“Ru-type ware” and unglazed firing bodies were unearthed from the Qingliang Temple Kiln in Baofeng County in the Henan province of China in 2014; the excavation site was close to the central firing area of the ancient Ru Kuan Kiln. Ru-type ware has several similar characteristics to Ru Kuan ware. Here, four samples of unglazed firing bodies, 10 samples of sky green Ru-type wares, and seven samples of sky green Ru Kuan wares were selected. The chemical compositions of 16 elements in body and glaze were measured by energy-dispersive X-ray fluorescence (EDXRF), and multivariate statistical analysis was conducted to determine the classification relations between Ru-type and Ru Kuan wares. Results indicate that the source of raw material and glaze formula of sky green Ru-type are different from Ru Kuan wares. For the sky green Ru-type ware, the raw materials for body is concentrated, and the glaze formula is stable, which conforms to the characteristics of official celadon.
The Ru Kuan kiln is one of the top five famous kilns of the Song Dynasty, and it was reserved for the Imperial Court. The Ru kiln produced an extremely rare type of pottery and porcelain, called Ru Kuan ware, that was only created for the Imperial Court in the Northern Song Dynasty (1). Ru Kuan ware pottery was famous for its delicate body quality, its exquisite workmanship, and its bright and smooth glaze color. The Ru Kuan kiln existed only approximately 20 years, prospering as a result of the continuous war between the Song and Jin Dynasty at the end of the Northern Song Dynasty. The result of this war led to the rarity of products handed down from ancient times and made this material even more precious.
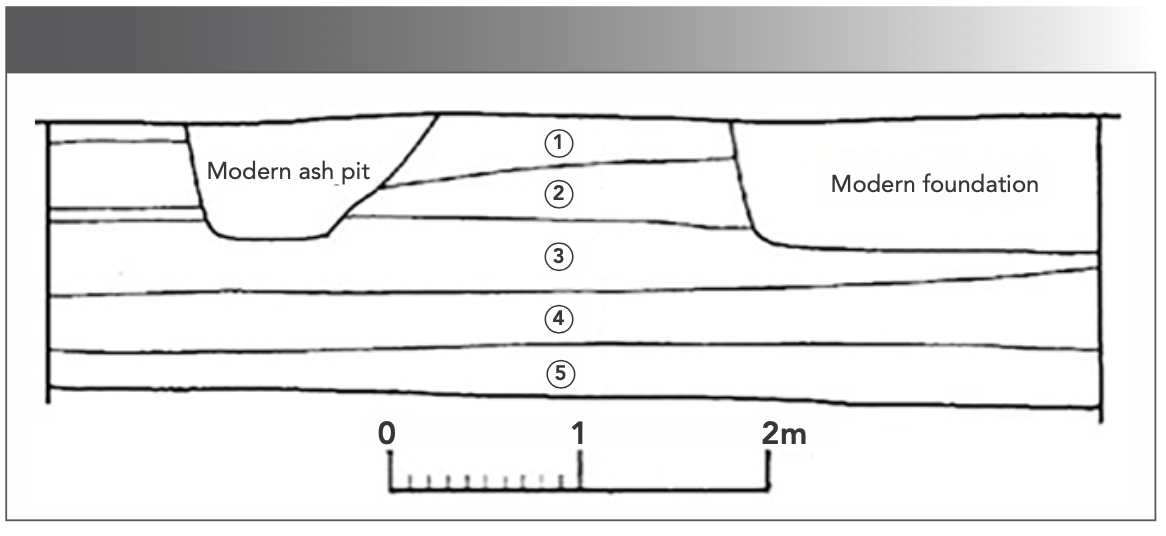
Qingliang Temple, located in Baofeng County in the Henan Province of China, was identified as the historical site of the official Ru kiln of the Song Dynasty in 2000 (2). From March 2014 to September 2014, the Henan Provincial Institute of Cultural Heritage and Archaeology conducted archaeological excavations in the central firing area and surrounding areas of the Ru Kuan kiln. A kiln furnace for unglazed firing and a small amount of unglazed firing shards were unearthed in the stratum of the nearest exploration pit (Figure 1) to the central firing area of Ru Kuan kiln (3). The stratigraphic relationship showed that the kiln furnace was built later than the mature period of the Ru Kuan kiln, which belonged to the late Northern Song or Jin Dynasty. In the same layer, another celadon-glazed porcelain was also unearthed; its body had fine and solid quality, with a greyish-white color and a little bit of the incense ash color normally associated with Ru Kuan ware. The celadon-glazed porcelain also contained the similar glaze color of sky green and grass green that was historically associated with Ru Kuan ware porcelain. Although the glaze color was bright and the glass texture is obvious, its jade texture was not as good as that of Ru glaze. This material was temporarily named “Ru- type ware” to distinguish it from Ru porcelain and also to acknowledge the several similarities that the two types of pottery possess (4). Ru-type ware is concentrated in the pit under the Yuan Dynasty layer (Layer 3) and the accumulation of the Jin Dynasty layer (Layer 4), and the stratigraphic age is also later than the mature period of the Ru Kuan kiln. Moreover, the Ru-type ware is large in size, with a regular shape, solid body quality, and a pure glaze color. Ru-type ware is also produced by full-glaze and pad firing. According to the standards for judging official and civil use, Ru-type ware should be for official use (5).
FIGURE 1: Stratigraphic profile of T85 exploration pit. Note: (1) Modern living and cushion soil layer, (2,3) Yuan and Ming Dynasties layer, (4) Jin Dynasty layer, and (5) late Northern Song Dynasty layer.
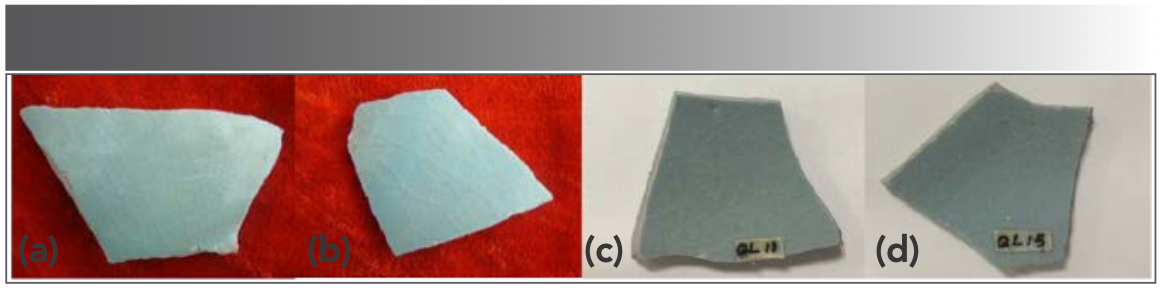
Amongst the celadon glaze color of Ru Kuan kiln, the sky green color is the most precious, as it represents the highest firing level of Ru Kuan ware. In this paper, the energy-dispersive X-ray fluorescence (EDXRF) technique is used to measure the chemical compositions of body and glaze samples of unglazed firing, sky green Ru-type ware, and Ru Kuan ware (Figure 2). The experimental results were subjected to multivariate statistical analysis to obtain the relationship between the raw material sources and glaze formulas of unglazed firing, sky green Ru-type ware and sky green Ru Kuan ware. Both Ru Kuan ware and Ru-type ware are very precious archaeological samples and there was no relevant research on Ru-type ware until now. This study lays the foundation for the preservation of samples and subsequent research studies. Furthermore, it provides a scientific basis for the firing age of Ru-type ware, identification of Ru Kuan and Ru-type wares, and research on the Ru kiln as well as the Northern and Southern Song Dynasties.
FIGURE 2: The figures of the typical samples. (a, b) Sky green Ru Kuan wares. (c, d) Sky green Ru-type wares.
Experimental
This experiment was conducted with the X-ray fluorescence spectroscopy elemental analysis system in the super-large sample room (Eagle III XLL μ-Probe) of the Institute of High Energy Physics Chinese Academy of Sciences. The spectrometer is equipped with a molybdenum tube and a 125 μm beryllium window that has an incident beam angle of 65° and an emergence angle of 60°. A silicon–lithium X-ray detector with a beam spot diameter of Ф = 1 mm, operating voltage of 40 kV, operating current of 250 mA, and the energy resolution of 160.3 eV, was used in this study because it can analyze the elements of 11Na–92U. To reduce the absorption of the low-energy secondary X-rays produced by the low-Z elements, the spectrometer was equipped with a vacuum chamber.
The relative position of the sample surface, the detector, and the light outlet were fixed, and the samples were positioned to the fixed focus of the analysis system accurately with the three-dimensional (3D) stepping motor, and high- and low- magnification charged-coupled device (CCD) cameras. The fundamental parameter (FP) method was used for nondestructive quantitative analysis of unglazed firing, sky green Ru-type and Ru Kuan ware body and glaze samples for 16 elements, such as sodium oxide (Na2O), magnesium oxide (MgO), aluminium oxide (Al2O3), silicon dioxide (SiO2), phosphorous pentoxide (P2O5), potassium oxide (K2O), calcium oxide (CaO), titanium dioxide (TiO2), manganese(II) oxide (MnO), and iron(III) oxide (Fe2O3). To calibrate the matrix effects and improve the accuracy of the data, we used a set of ceramic reference materials for EDXRF by the Institute of High Energy Physics. The data were processed using the program version 32, which was provided with the equipment. Through discriminant analysis and hierarchical cluster analysis (HCA) in multivariate statistical analysis, the relationship between the chemical components was studied (6). The classification of unglazed firing, sky green Ru-type, and Ru Kuan ware body and glaze samples were obtained.
Four samples of unglazed firing, 10 samples of sky green Ru-type ware, and seven samples of sky green Ru Kuan ware shards were selected for this experiment. The shards were provided by the Henan Provincial Institute of Cultural Heritage and Archaeology, and those samples were from accurate and reliable sources. The selection of samples is shown in Table I. The glaze color of Ru-type and Ru Kuan wares mentioned in this paper is sky green and will not be repeated hereinafter.
Results and Discussion
The chemical composition of 16 elements of body and glaze samples of unglazed firing, Ru-type, and Ru Kuan ware was measured by EDXRF. The contents of seven elements, including Al2O3, SiO2, and K2O, are listed in Tables II and III.
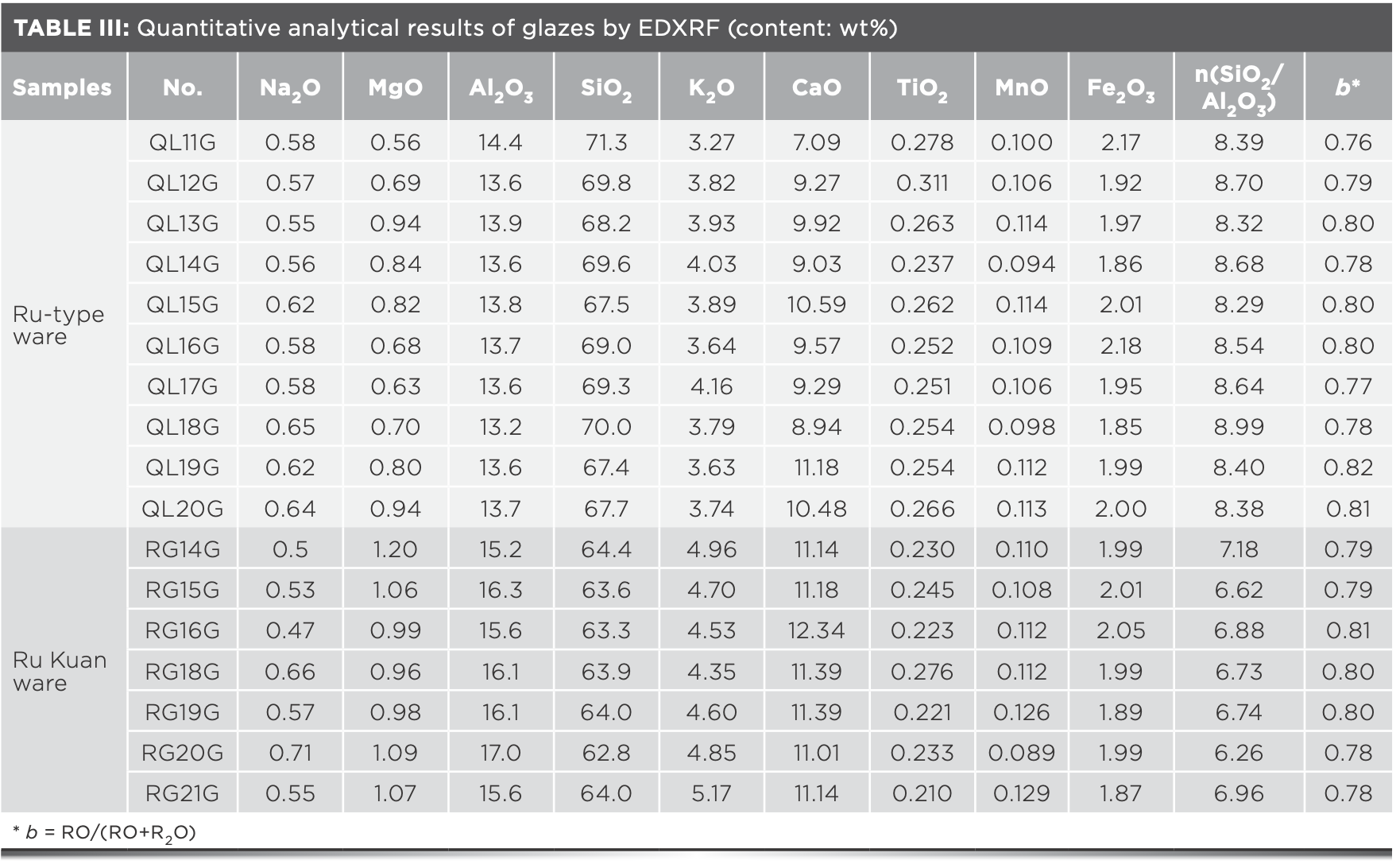
Body Raw Materials by Discriminant Analysis
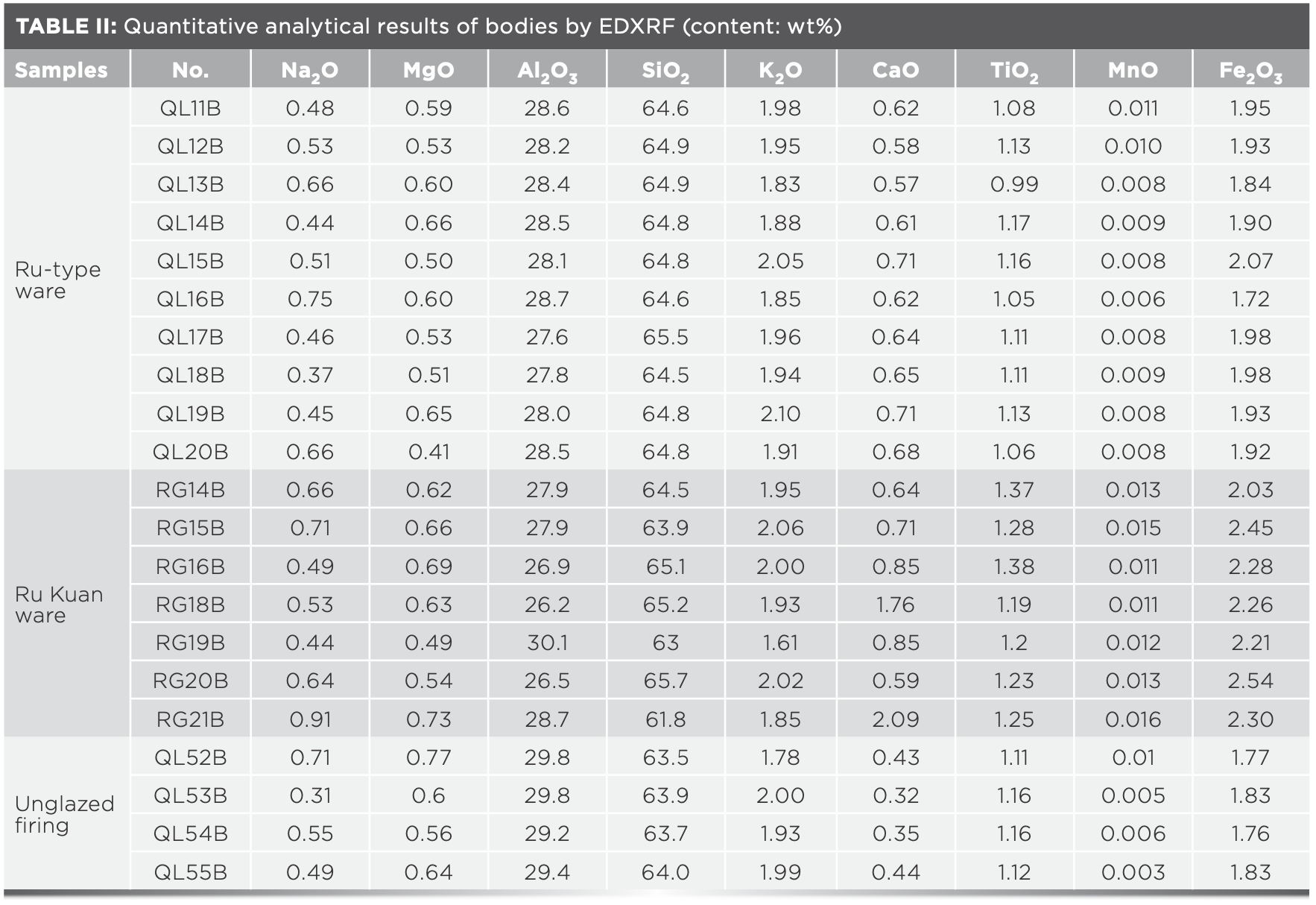
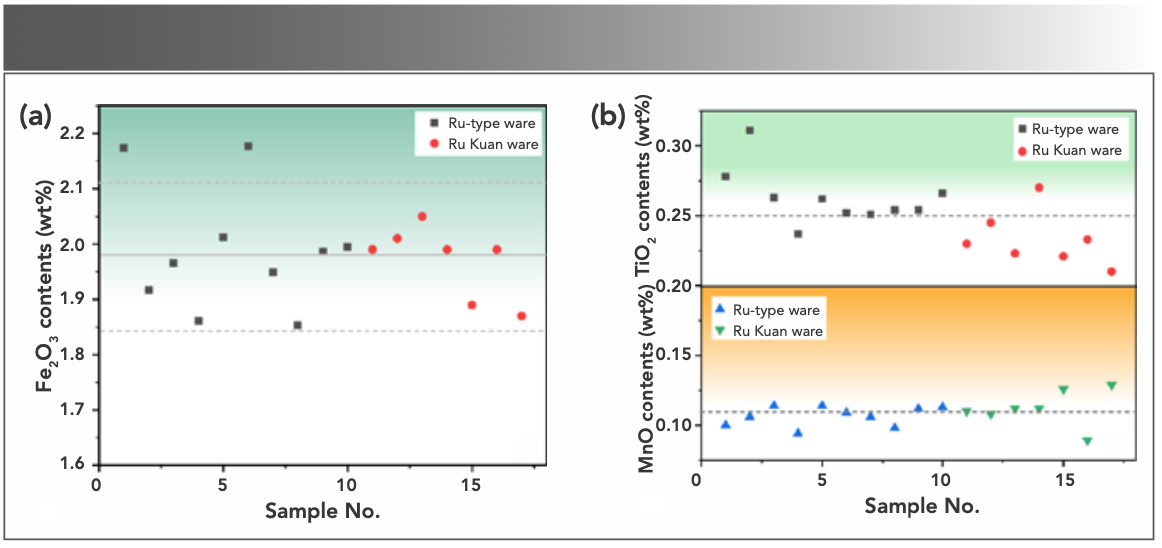
The main raw material of celadon body in northern China is clay, which is comprised of many minerals. The oxide contents in clay minerals would vary in different geological formation conditions and include a small amount of alkaline-earth metal oxides, such as Fe2O3 and TiO2, in addition to hydrous aluminosilicate (7). Fe2O3 and TiO2 are usually dispersed in clay as fine grains, and their combinations combine and form compounds, such as FeO·TiO2, 2FeO·TiO2, and Fe2O3·TiO2 at high temperatures; thus, making the bodies show different shades of gray. The Fe2O3 content was taken as the horizontal coordinate and the TiO2 content as the vertical coordinate. A 2D dispersion analysis chart was made, as shown in Figure 3. Table II and Figure 3 show that the contents of Fe2O3 and TiO2 of Ru Kuan ware bodies (Fe2O3: 2.21~2.54 wt% and TiO2: 1.2%~1.38 wt%), except for RG14B, were higher than those of Ru-type ware (Fe2O3: 1.72~2.07 wt% and TiO2: 0.99~1.17 wt%) and unglazed firing (Fe2O3: 1.76~1.83 wt% and TiO2: 1.11~1.16 wt%) bodies. This finding indicates that the source of bodies of Ru Kuan ware is different from that of Ru-type ware and unglazed firing, which may have used clay materials with higher hematite, ilmenite, or rutile minerals. The RG14B sample has a higher TiO2 content than unglazed firing and Ru-type ware, and the Fe2O3 content is only slightly lower than that of one Ru-type ware sample (QL15B). RG14B shows that the contents of Fe2O3 and TiO2 of Ru Kuan ware bodies are higher than that of the Ru-type ware and unglazed firing bodies. Ru-type ware and Ru Kuan ware can be classified by the iron and titanium contents of bodies.
FIGURE 3: 2D dispersion analysis of Fe2O3 and TiO2.
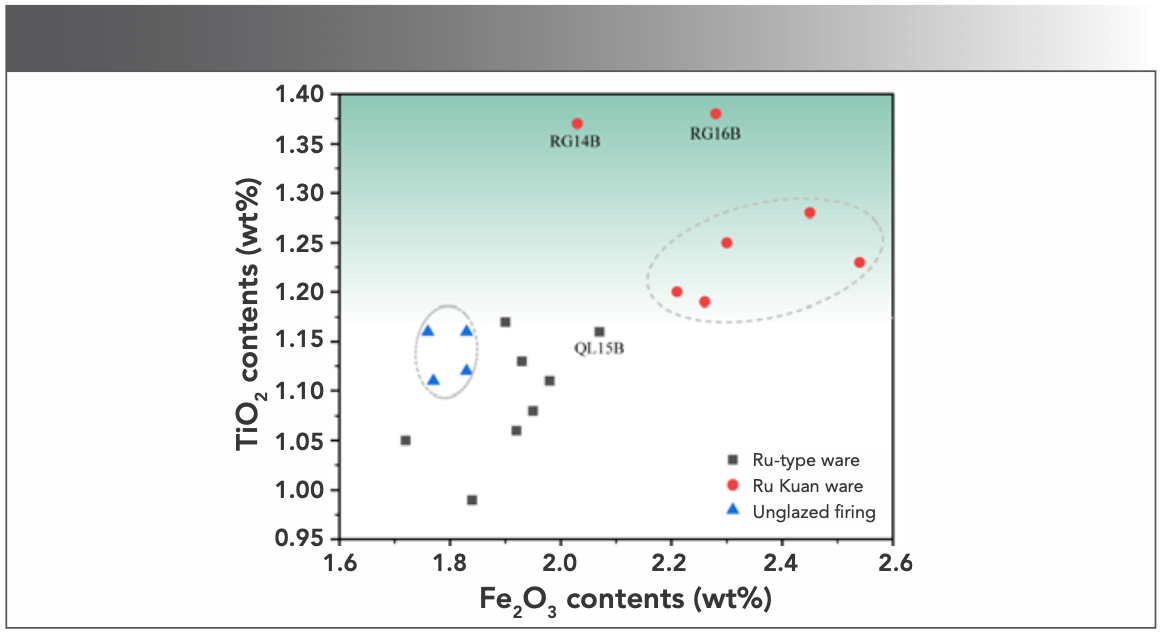
Although the TiO2 content range of Ru-type ware and unglazed firing body overlaps to some extent, the overall content of Fe2O3 of Ru-type ware was higher than that of unglazed firing. This result shows that the two may have differences in the choice of raw materials, but they may have the same sources of raw materials. However, the content of iron minerals is reduced in the raw material treatment processes, such as elutriation, in the preparation process of unglazed firing. The differences in Fe2O3 and TiO2 content imply that the level of raw material composition and origin result in that the body of Ru Kuan ware is incense ash color, Ru-type ware is greyish white, and unglazed firing is white.
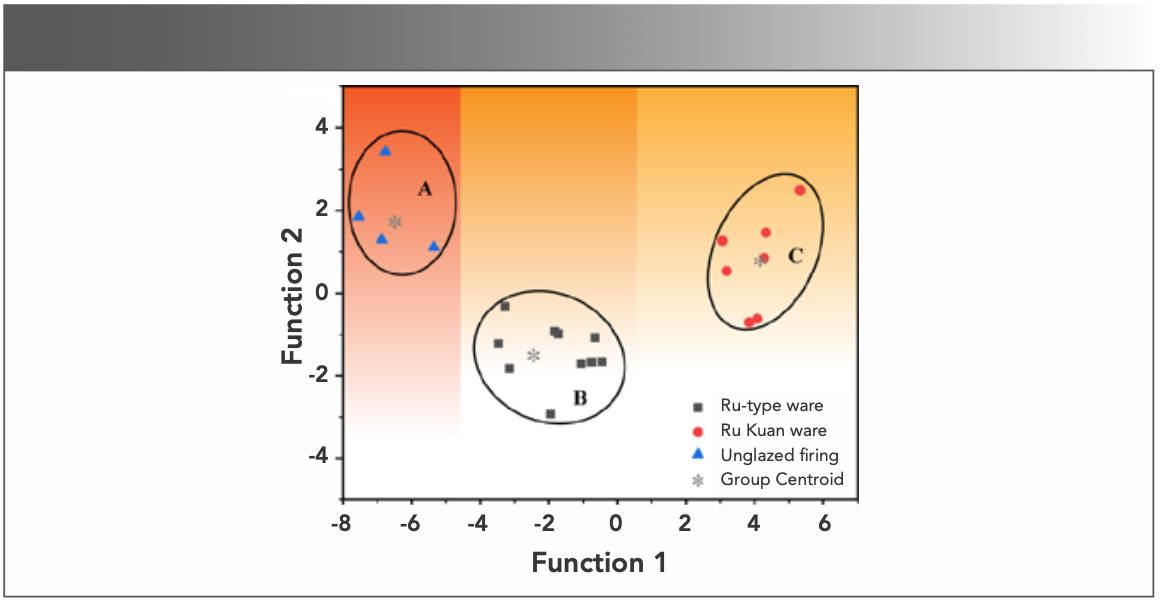
Discriminant analysis can be used to train the discriminant function based on the different eigenvalues and the category to which the research object is known to belong; when a sample with an unknown category is entered, the sample is classified according to the similarity level between it and the discriminant function (8). In this experiment, Fisher discriminant method was used to analyze the content of all chemical components of 25 body samples. Figure 4 shows a two-dimensional (2D) visual classification scatter diagram. The body samples of unglazed firing, Ru-type ware and Ru Kuan ware are concentrated in three areas of A, B, and C, respectively. This condition is ideal and indicates that the raw material sources of the three kinds of body samples are completely different. Although unglazed firing and Ru-type ware belong to the same excavation layer, their sources of body materials differ, which is caused by differences in the origin of raw materials or firing batches. The two categories differ from those of Ru Kuan ware, which shows that the two differ in terms of body material selection for the porcelain made in the Ru Kiln. The Ru-type ware data points in area B and the Ru Kuan ware data points in area C are concentrated, indicating that sky green Ru-type ware body is strictly controlled in raw material selection and craftsmanship, which proves it was used for official occasions.
FIGURE 4: Discriminant analysis of bodies.
Glaze Formula by Dispersion Analysis and Hierarchical Cluster Analysis
The content of oxides of Ru-type and Ru Kuan ware glazes is shown in Table III. After the molar coefficient of alkaline oxide (RO+RO2) is adjusted to 1, the Seger formulas of Ru-type and Ru Kuan ware glazes are 1.1 and 1.2, respectively.
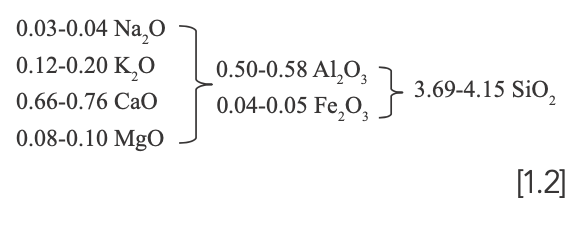
Dispersion Analysis
Fe2O3, MnO, and TiO2 are the colorants of ceramic glaze, with iron being the main coloring element of celadon glaze in northern China (9). Figure 5a shows the 1D dispersion analysis diagram of the Fe2O3 content. As observed, the overall Fe2O3 content of Ru-type and Ru Kuan ware glazes is at the range of 1.85~2.18 wt%, which shows that the iron mineral content in the glaze formula of the two is close. Manganese and titanium are not the main coloring elements, but they have certain influence on the hue of celadon. MnO and TiO2 are brought with the clay along with Fe2O3. Figure 5b shows the 1D dispersion analysis diagram of MnO and TiO2. The MnO contents of Ru-type and Ru Kuan ware glazes fluctuate around 0.11 wt%, and the content of each glaze sample is close to that of others. On the whole, the TiO2 content of Ru-type ware is higher than that of Ru Kuan ware glazes, which indicates that the mineral content of titanium of Ru-type ware glaze is higher, or some ilmenite is involved in the glaze ratio.
FIGURE 5: (a) 1D dispersion analysis of Fe2O3. (b) 1D dispersion analysis of TiO2 and MnO.
The content of SiO2 and Al2O3 accounts for 81~85.7 wt% and 78.9~80.1 wt% of the total oxides of Ru-type and Ru Kuan ware glaze samples, respectively. The two oxides with a great deal of fluxing agent chemically react to form glaze at high temperature (9), and the molar ratio of the two contents can intuitively characterize the changes in glaze formula and process technology (10). Figure 6 shows the dispersion analysis of the SiO2 and Al2O3 contents and the silica-alumina ratio for Ru-type and Ru Kuan ware glazes. The molar silica-alumina ratios are listed in Table III. Except RG20G, Ru Kuan ware is concentrated in area C, and Ru-type ware is concentrated in area D. The comparison shows that Ru Kuan ware glaze has high aluminum and low silicon content, whereas Ru-type ware glaze has high silicon and low aluminum element content, indicating that Ru-type ware is different from Ru Kuan ware regarding its glaze formula. Ru Kuan ware is glazed with agate. A rich agate ore is observed near the site of the Qingliang Temple Kiln (11). Agate is a cryptocrystalline mineral of SiO2, which is added into the glaze to achieve the similar function to quartz. The SiO2 content of Ru-type ware glaze is higher than that of Ru Kuan ware glaze, which might result from the higher quartz content in the glaze formula, or the similar glaze technology with agate in Ru Kuan ware to some extent, but the amount used is higher than that of Ru Kuan ware glaze. Al2O3 is mainly brought by clay and feldspar, and the content of Al2O3 of Ru Kuan ware glaze is higher than that of Ru-type ware. Therefore, the Ru Kuan ware used high aluminous clay in the formula, and a larger amount of feldspar is found in the formula. Although RG20G is not in area C, its SiO2 and Al2O3 contents are consistent with the characteristics of high aluminium and low silicon contents of Ru Kuan ware.
FIGURE 6: 2D dispersion analysis of SiO2 and Al2O3. (insert) 1D dispersion analysis of n(SiO2/Al2O3).
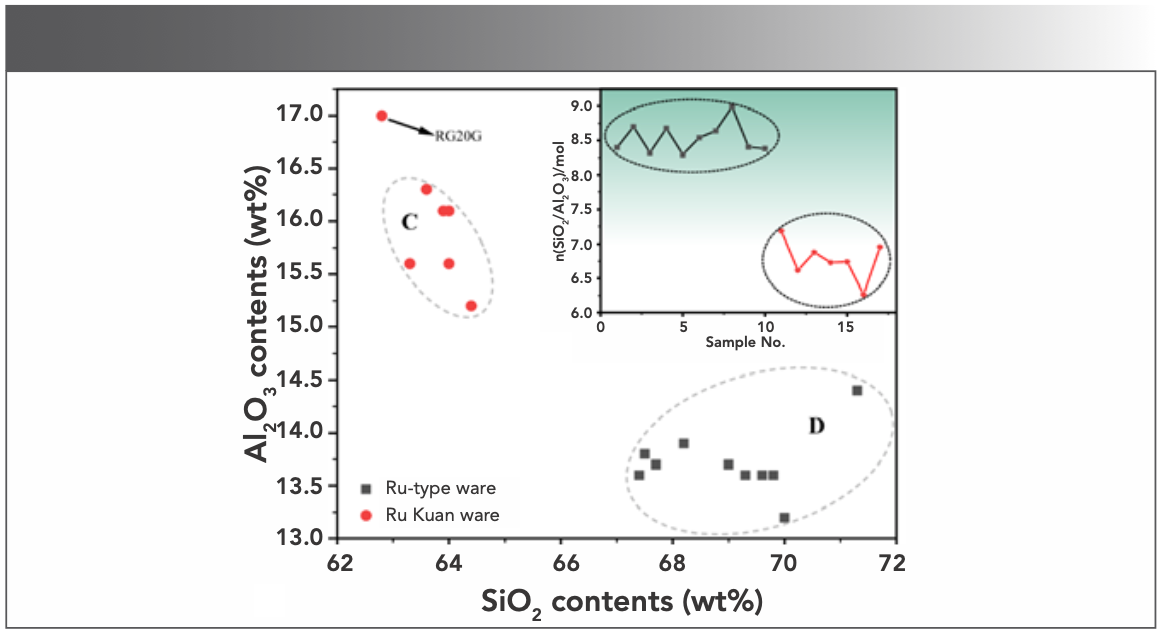
Table III and Figure 6 show that the molar radio n(SiO2/Al2O3) of Ru-type ware glaze ranges from 8.29 to 8.99, higher than that of Ru Kuan ware glaze with the range from 6.26 to 7.18, which also shows that the glaze formulas of the two differ. Moreover, the silicon:aluminium ratio fluctuation range of the two is relatively small, which implies that the glaze formula is relatively stable and also proves that Ru-type ware has been used for official occasions.
The traditional high-temperature glaze in China has always been glazed by limestone and plant ash, amongst which the fluxing agents, such as CaO, MgO, and K2O, can reduce the melting temperature of glaze. Their contents and types also have a decisive influence on the melting temperature and appearance characteristic of glaze. CaO in glaze mainly comes from limestone and calcite (main ingredient is CaCO3), whereas MgO mainly comes from dolomite (CaCO3·MgCO3). Figure 7a shows a 2D dispersion analysis of calcium and magnesium oxides. A positive correlation is observed between the CaO and MgO contents of Ru-type and Ru Kuan ware glazes, which may be associated with the dolomite composition of the initial mixed glazes. In addition, the contents of CaO and MgO of Ru Kuan ware glaze are higher than those of Ru-type ware glaze, which may be caused by higher content of anorthite, limestone, and dolomite in glaze.
FIGURE 7: (a) 2D dispersion analysis of CaO and MgO, (Insert) showing CaO and MgO content of various sample numbers. (b) Box plot of K2O and P2O5.
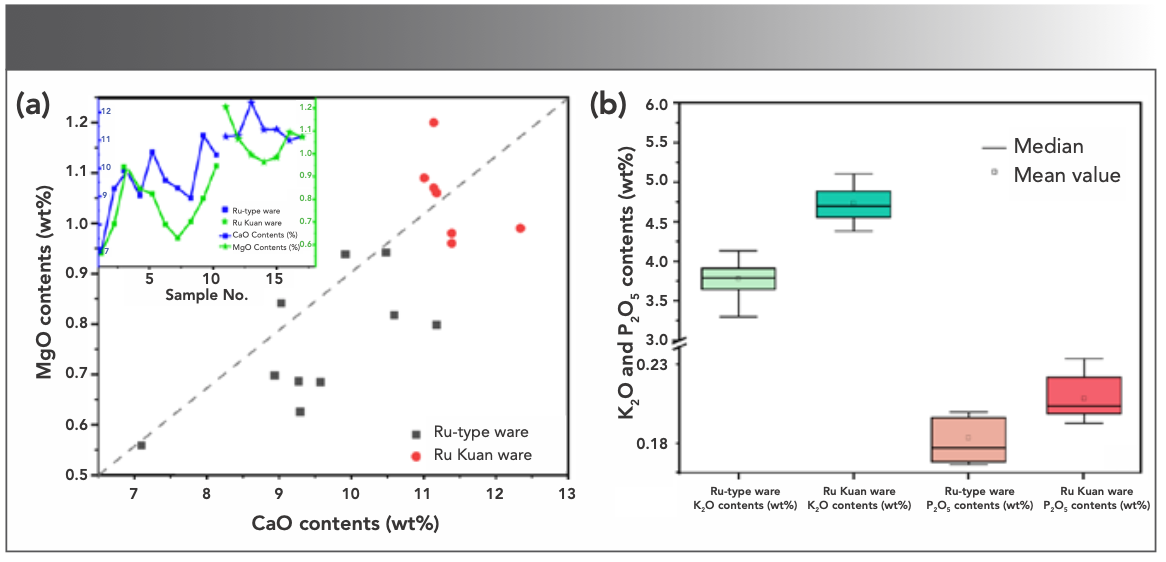
K2O as a fluxing agent is mainly from potassium feldspar, and ice cracks can be produced on the glaze when the content is high because of the larger coefficient of expansion. P2O5 as a kind of opacifier that can result in varying degrees of opacified texture on the glaze surface. P2O5 basically comes from plant ash, and the content of varies considerably from species to species. Figure 7b shows the box plot of K2O and P2O5. The contents of K2O and P2O5 of Ru Kuan ware glaze are higher than those of Ru-type ware glaze on the whole. Therefore, Ru Kuan ware adopts the glaze formula with higher potassium feldspar ratio, while Ru-type ware is glazed with plant ash with less P2O5 content different from Ru Kuan ware.
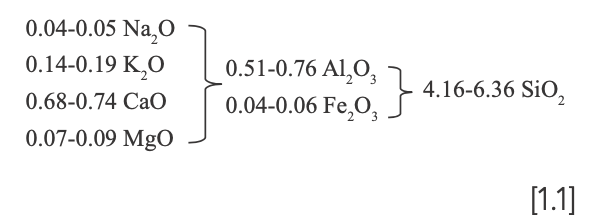
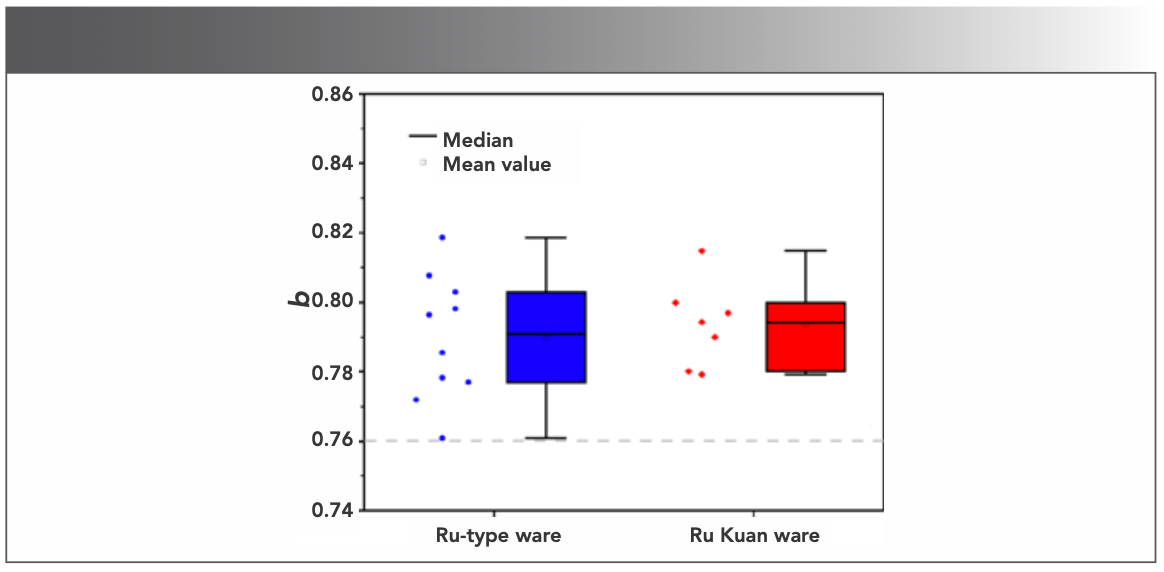
Hongjie Luo (12) proposed a reference standard for classifying Chinese ancient porcelain glaze based on the coefficient b of wood ash glaze formula, which is the molar ratio of alkali-earth metal oxide (RO) to the sum of alkali metal oxide (R2O) and alkali-earth metal oxide. This classification consists of three groups: calcium glaze (b ≥ 0.76), calcium–alkali glaze (0.76 > b ≥ 0.5) and alkali–calcium glaze (b < 0.5). The calculated b values of each sample are listed in Table III, and the box plot is shown in Figure 8. The results show that the b values of Ru-type and Ru Kuan ware glazes are all above the dividing line b = 0.76, which indicates that both are high calcium glazes. The b values of the two are relatively close (the range of b values is 0.76~0.82 for Ru-type ware and 0.78~0.81 for Ru Kuan ware), which indicates that Ru-type ware may follow the glaze-making technology of Ru Kuan ware to some extent. With the small fluctuation range of the relatively stable glaze formula, Ru-type ware contains the characteristics of official celadon.
FIGURE 8: The b values of Ru-type ware and Ru Kuan ware.
Hierarchical Cluster Analysis (HCA)
HCA classifies the samples into groups and subgroups according to the similarity and correlation of the samples (13). The HCA of Ru-type and Ru Kuan ware glazes is shown in Figure 9, and can be divided into two categories.
FIGURE 9: The HCA of Ru-type ware and Ru Kuan ware.
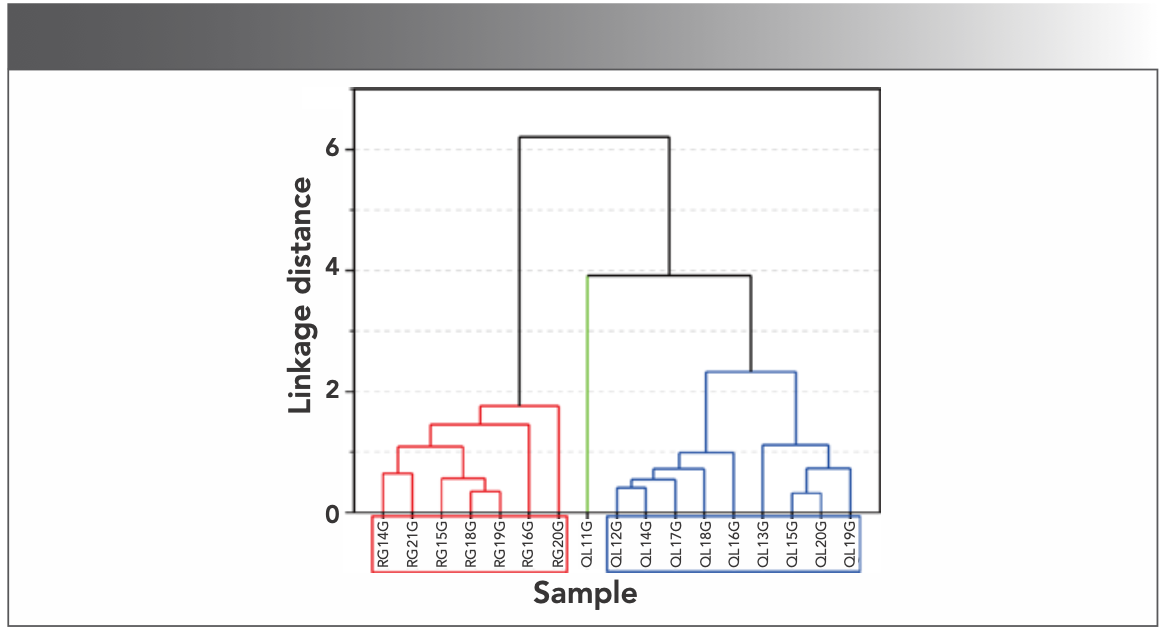
The first category includes all Ru Kuan ware glaze samples from RG14G to RG20G, indicating that the formula of Ru Kuan ware glaze in this category is relatively stable.
The second category includes all Ru-type ware glaze samples from QL11G to QL19G. The formula of Ru-type ware glaze in this category has also become mature and relatively stable, which conforms to the properties of the official glaze formula. Although sample QL11G is slightly far from the nine other samples of Ru-type ware, it can still be classified into one category. On the whole, the formulas of Ru-type and Ru Kuan ware glazes are completely different.
Conclusion
The raw material sources of unglazed firing, sky green Ru-type ware and sky green Ru Kuan ware bodies differ. Although unglazed firing and sky green Ru-type ware belong to the same excavation layer, their sources of body materials differ. The body of sky green Ru Kuan ware is made of clay raw materials with minerals, such as high hematite, ilmenite, or rutile.
Sky green Ru-type ware is different from sky green Ru Kuan ware in terms of glaze formula. The high content of quartz in sky green Ru-type ware glaze and the high content of feldspar in sky green Ru Kuan ware glaze show that the element composition characteristics of the former are a high ratio of silicon and low aluminium, while those of the latter are high ratio of aluminium and low silicon. Moreover, the sky green Ru Kuan ware glaze is glazed of plant ash with high P2O5 content, and the glaze contains higher limestone, dolomite and potassium feldspar. The content of coloring elements iron and manganese of the two glazes are close, whilst the content of TiO2 of sky green Ru Kuan ware is higher. Ilmenite is involved in the glaze ratio.
The glaze and body of sky green Ru-type ware are strictly controlled in raw material selection and craftsmanship, which proves that it has been used for official occasions. The sky green Ru-type and Ru Kuan ware glazes are high calcium glazes with the relatively close b values and small fluctuation. The glaze formulas of the two are stable and concentrated, which belong to the characteristics of official celadons.
The sky green Ru-type and Ru Kuan wares are classified by the Fe2O3 and TiO2 contents of bodies and the SiO2, Al2O3, and P2O5 contents of glazes by EDXRF.
Acknowledgments
This work was supported by National Natural Science Foundation of China 11275173 and 11975210.
References
(1) W. Li, J. Li, Z. Deng, J. Wu, and J. Guo, Ceram. Int. 31, 487–494 (2005).
(2) W.J. Zhao, Z.J. Wu, G.X. Li, M. Guo, J.Z. Xie, X.K. Lu, X.M. Sun, S.L. Feng, and M.S. Guo, J. Chinese Ceram. Soc. 35, 1556–1560 (2007).
(3) H. Zhao, Archaeological Discoveries of Ru Kiln in Baofeng Qingliang Temple, Chinese Ru Kiln academic proceeding (Henan People’s Publishing House, Zhengzhou, China, 2017).
(4) B. Wu, W.J. Zhao, S.L. Feng, H. Zhao, X.M. Sun, and M.S. Guo, J. Ceram. 39, 588–591 (2018).
(5) X.M. Sun, H. Zhao, J.L. Zhao, T.J. Wang, Z.J. Niu, W.B. Wang, and H.W. Lu, Huaxia Archaeology 1, 49–60 (2019).
(6) P. Mukhopadhyay, Multivariate Statistical Analysis (World Scientific, Singapore, 2008).
(7) I.O. Materials, Ceramics Technology (Materials Information, ASM International, Novelty, Ohio, 1996).
(8) W.S. Rayens, Technometrics 35, 324–326 (2010).
(9) F.K. Zhang, Science of Chinese Ancient Ceramics (Shanghai People’s Fine Arts Publishing House, Shanghai, China, 2000).
(10) Y.Z. Ding, J.Y. Hou, H. Li, J.M. Wu, and X.M. Sun, China Ceram. 55, 59–63 (2019).
(11) J.Z. Li, History of Science and Technology of China (Ceramic Volume) (Science Press, Beijing, China, 1998).
(12) H.J. Luo, Chinese Ancient Ceramics and Mutivariate Statistical Analysis (China Light Industry Press, Beijing, China, 1997).
(13) R.A. Ikeoka, C.R. Appoloni, M.A. Rizzutto, and A.M. Bandeira, Microchem. J. 138, 384–389 (2018).
Bo Wu, Weijuan Zhao, Dan Zhao, and Xiaomin Liu are with the School of Physics and Microelectronics at Zhengzhou University in Zhengzhou, China. Songlin Feng and Xiangqian Feng are with the Institute of High Energy Physics Chinese Academy of Sciences in Beijing, China. Hong Zhao is with the Henan Provincial Institute of Cultural Heritage and Archaeology in Zhengzhou, China. Direct correspondence to Weijuan Zhao: zwj@zzu.edu.cn.●

AI Shakes Up Spectroscopy as New Tools Reveal the Secret Life of Molecules
April 14th 2025A leading-edge review led by researchers at Oak Ridge National Laboratory and MIT explores how artificial intelligence is revolutionizing the study of molecular vibrations and phonon dynamics. From infrared and Raman spectroscopy to neutron and X-ray scattering, AI is transforming how scientists interpret vibrational spectra and predict material behaviors.
Advancing Corrosion Resistance in Additively Manufactured Titanium Alloys Through Heat Treatment
April 7th 2025Researchers have demonstrated that heat treatment significantly enhances the corrosion resistance of additively manufactured TC4 titanium alloy by transforming its microstructure, offering valuable insights for aerospace applications.