Temporary Online FT-IR Spectroscopy for Process Characterization in the Chemical Industry
Case studies involving fouling and product quality illustrate the effective use of this method.
The focus of this paper will be on the use of temporary online Fourier-transform infrared (FT-IR) spectroscopy in the chemical industry including two case-study applications involving fouling and product quality. These case studies will be followed by a discussion of the use of temporary online FT-IR analysis enabling process optimization.
The Dow Chemical Company (Midland, Michigan) is the world's largest integrated chemical company utilizing a wide variety of unique chemistries in the production of both basic and specialty chemicals. The diversity of chemical processes and the interrelationships of production facilities in the integrated chemical complex provide significant opportunities for process optimization. Fouling, corrosion, plugging, or out-of-specification product occurrences in one manufacturing plant can have negative effects on the operation of one or more downstream facilities. Accurate and representative data for process characterization is a key factor in making informed decisions regarding the resolution of process problems. The information-rich nature of optical spectroscopy and, in particular, Fourier-transform infrared (FT-IR) spectroscopy is uniquely suited for rapid and continuous online process characterization.
Sometimes grab samples of the process that are analyzed in the laboratory provide sufficient data for process characterization. However, the nature of a process stream frequently makes collecting representative grab samples difficult. Gas-phase samples, reactive samples, and samples under elevated (or depressed) temperature or pressure are a few examples of samples that are particularly difficult to collect and analyze by laboratory analysis. Streams where trace (for example, low parts per million) levels of water or carbon dioxide are the analytes of interest are also a particular challenge for grab sample laboratory analysis because concentration levels are easily influenced by atmospheric composition. Safety issues must also be considered when collecting samples of highly toxic or explosive materials such as phosgene, acrylates, ethylene oxide, hydrogen chloride, or isocyanates. Depending on the duration and extent of required toxic material analyses, properly designed continuous online analysis can help mitigate associated safety issues. Additionally, when the process event of interest is transient occurring over a short time-frame, occurring at unknown intervals, or if the process is not at steady-state, a continuous online analysis of a representative sample provides a richness of insight into the process that would not be achievable by the infrequent glimpses into the process that is attainable by grab sample analysis. Lastly, continuous analyses are an invaluable tool during new process development and process scale-up due to the ability to thoroughly characterize the process.
Of the available analytical tools for temporary online process characterization, FT-IR is among the most powerful. FT-IR is a fast technique providing fundamental vibrational information for the components in the stream and allowing the capture and retention of information-rich spectra. The raw data obtained from FT-IR are often referred to as "traceable" because analysts can refer to primary and secondary standards corresponding to the spectral information from the process sample. Analysts may also examine the raw spectral data for interferences that have not been present previously or do not match the anticipated stream composition. A variety of chemometric modeling techniques (1) are available to obtain quantitative information, trending information, or simple qualitative stream composition analysis from the raw spectra depending on the purpose and requirements of the particular application.
The insights and data obtained from continuous online FT-IR can be applied to optimize process control models, validate the consistency of production quality, or inform development of robust and reliable permanent process analyzer applications. The following case studies focus on process control optimization and product quality.
Experimental
Before discussing case studies it will be useful to highlight a few basic considerations for installing a FT-IR system in a process environment. Of course, spectrometer setup, operation, and data processing must be appropriately specified for a given application, but unless the sample is reliably and safely delivered to the spectrometer the analysis details are mute. Process analyzer installation and methods can be complex (2), but two major design considerations for temporary analysis are safety and sample system design. The first to consider is safety. Although some safety concerns associated with handling samples for laboratory analysis are mitigated by using a process analyzer and eliminating routine grab sampling, a distinct set of other safety related hazards must be addressed. Of primary concern is assuring area classifications are met to mitigate explosion or exposure risks. In the first case study, the stream was comprised mostly of ethylene and the area classification issue was of critical importance. Because the FT-IR system that was used was not classified for a Class 1 Division 2 area, operating procedures were developed designating attended operation with appropriate area lower explosion limit (LEL) monitoring and emergency shutdown plans should the LEL monitor indicate the presence of a flammable atmosphere. A picture of the field setup of the analyzer is shown in Figure 1. On days when there was a threat of rain, a canopy was placed over the whole system. The preferred option would have been to package the spectrometer in a z-purged analyzer enclosure. Cost and timing constraints prevented the preferred path of C1D2 packaging for the analyzer in the ethylene analysis case study. Figure 2 shows the analyzer packaged for the product quality case study where the analyzer was installed in the field and operating under nonattended operation 24/7. In the product quality case study, the location was a nonclassified area, but the stream composition was highly toxic and corrosive. The analyzer enclosure was exposed to the elements and vortex coolers were used to control the temperature inside the enclosure.

Figure 1: Temporary setup of FT-IR analyzer for analysis of the ethylene stream in case study 1 for use with attended operation only.
The second set of design considerations revolve around sample systems and sample handling panels. Having a continuous online analysis is not useful if the analyzer is not provided with a consistent and representative process sample for analysis. Sample systems are an integral part of the success or failure of a process analytical system (3). FT-IR and spectroscopic techniques in general demand much simpler sample systems than required by typical process gas chromatography systems, but fundamentals such as flow and pressure as well as avoiding condensation, particulates, and plugging are still essential. Key methods for liquid- and gas-phase sample systems for FT-IR implementation are different. Here, the focus is on gas-phase FT-IR as this technique has repeatedly been demonstrated to meet the temporary online stream characterization requirements in a variety of Dow processes. When it comes to liquid-phase analysis, attenuated total reflectance (ATR) FT-IR techniques are occasionally used, but probe fouling and lack of ability to monitor trace levels can be limiting and the use of a near-infrared or Raman system may be preferable. For gas-phase process streams, long-pathlength FT-IR analysis is a workhorse for stream characterization. The two systems that were used in the case studies discussed below are the MKS 2032 MultiGas FT-IR system (MKS Instruments, Andover, Massachusetts) and the Gasmet DX4000 FT-IR system (Gasmet Technologies Oy, Finland). Other FT-IR systems could also be utilized, but portability and 24/7 continuous data acquisition are key requirements.

Figure 2: Temporary setup of FT-IR analyzer in case study 2 for continuous 24/7 data collection.
Case Study — Fouling
The first case study is an application in a plant trying to solve plugging and fouling events that have been occurring regularly over multiple years. The stream was primarily ethylene with other C2–C8 hydrocarbons and unknown low levels of acrylic acid monomer, water, and other small organics thought to be present in concentrations less than 1 mol%. It was known that the substance plugging the equipment at the facility was polyacrylic acid polymer and that minimizing the presence of acrylic acid and water in the stream would minimize the fouling. Several process control changes had been tried throughout the years, based purely on Aspen process models (Aspen Technology, Inc., Burlington, Massachusetts), but the plugging issues persisted. The unknown was how various process operating conditions in surrounding portions of the plant impacted the residual levels of acrylic acid and water in the region where fouling occurred. Attempts to validate the assumptions used for the process models using aluminum oxide moisture measurements failed because the aluminum oxide moisture analyses used were infrequent, slow to equilibrate, suffered from calibration drift because of stream components fouling the sensor, and were not reproducible. Additionally, the aluminum oxide sensor only provided data on the moisture component, not the acrylic acid concentration or any of the other hydrocarbons or organics that were suspected to be present.
To achieve a fast response, quantitative results, low detection limits, and multicomponent analysis, a long-path FT-IR was installed at the process location with a sample line comprising 50 ft of ¼-in. diameter heat traced tubing to minimize condensation or changes in the sample between the process pipe and the analyzer. The process sample was returned to the plant's vacuum vent line. This process drop allowed for continuous flow at the rate of ~2–3 L/min and a constant pressure in the FT-IR sample cell. The sample cell pathlength was 5.11 m and 0.5 cm-1 spectral resolution was used. Figure 3 shows an example spectrum of 100% ethylene as well as water and acrylic acid in the concentration range of interest. Figure 3 also provides a process spectrum clearly indicating that both water and acrylic acid are present in the process ethylene. A total of six components were monitored in the ethylene process stream, including the water and acrylic acid.
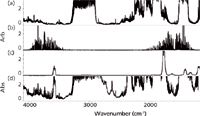
Figure 3: Spectra of (a) 100% ethylene, (b) 500 ppm water, and (c) 932 ppm acrylic acid, and (d) a process spectrum at 150 °C and 1 atm with a 5.11-m pathlength at 0.5-cm-1 resolution and a 1-min collection time.
A spectral data point was collected every minute to capture process fluctuations. A designed set of experiments was implemented that systematically changed the plant's operating conditions within acceptable ranges while levels of acrylic acid and water, among other components, were monitored. Figure 4 includes process trend comparisons between just two of the process condition experiments showing a better than threefold difference in water concentration while having negligible effects on acrylic acid levels. The fluctuations within each of the given trials also correlated with specific process parameters and provided insight into process stability. Other tested process conditions demonstrated decreases in residual acrylic acid.
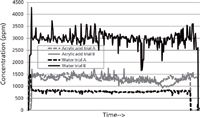
Figure 4: Acrylic acid and water trends during two different process trials showing significant impact on the water concentrations between the two sets of process conditions.
The key information learned from continuous FT-IR data of acrylic acid and water, as well as the other components, in comparison with known process control parameters was identification of specific process parameters that enabled reduction in acrylic acid and water without adversely affecting quality or other process operations. The analytical results were used to optimize the process control model based on Aspen simulations. The new process model was implemented in the plant for control. Before implementation of the model, fouling occurred every 1–2 months causing 2–3 days of downtime per incident. Since implementation of the model more than 1 year ago there has not been a shutdown caused by the fouling problem. This case study is an excellent example of FT-IR spectroscopy being used for continuous online analysis to improve process operation by providing real-time data for process correlation. The plant's issue was solved without investment in installation and maintenance of a permanent FT-IR (or any other) process analyzer.
Case Study — Product Quality
A second case study, in which gas phase FT-IR was applied to characterize a process, was for the purpose of product quality optimization. In this case, the goal was product purity. The stream was very simple with a single component being greater than 99.99% pure with contaminants of interest for continuous analysis, carbon monoxide (CO) and carbon dioxide (CO2), being present at levels below 1 ppm under normal conditions. The major challenge with analyzing CO2 by grab sample and laboratory analysis is atmospheric contamination of the sample. By using a continuous online sample system that is free of leaks, a more representative sample is achieved. In this particular case study, the plant was interested in increasing the purity of its hydrochloric acid (HCl) product. The FT-IR data were used to validate process improvements with regard to minimizing the CO2 and CO content of the product.
Two different FT-IR systems were applied for the sub-part-per-million analysis of CO and CO2 in HCl because of changing equipment availability. Both used a 5-m pathlength multipass cell, a 1-min collection time, and a 60 °C cell temperature, and both operated with sample pressures slightly below atmospheric pressure. The primary difference between the two FT-IR systems was spectral resolution. One system was operated at 0.5-cm-1 resolution and the other at 8 cm-1. Both used modified classical least squares (CLS) algorithms for quantitative concentration determination. There were advantages and disadvantages of operating under the different resolutions (5), but both demonstrated capability for the analysis.
The lower-resolution system required greater attention to the CLS model prediction because of spectral overlap between deuterium chloride (DCl) and CO. The ro-vibrational spectra of HCl is well known to have rotational structure from both the H35Cl and H37Cl isotopes. With the natural abundance of deuterium being 156 ppm, the spectrum of anhydrous HCl also contains rotational structure from the υ=1←0 band of D35Cl and D37Cl centered at 2145.163 cm-1 (7) and overlaps the fundamental CO stretch region. Figure 5 shows the overlap of DCl and CO at 0.5 cm-1 and 8 cm-1 resolution. With the spectrometer system at 0.5 cm-1 resolution, the best quantitative solution was to rely on CO transitions located in the DCl Q-branch gap. Specifically, the three CO transitions at 2094.8 (P(12)), 2090.6 (P(13)), and 2086.3 cm-1 (P(14)) were used for the CO analysis on the 0.5 cm-1 resolution spectrometer. A modified CLS method based off of peak areas was used for quantitative prediction with the 0.5-cm-1 system. At 8-cm-1 resolution, a CLS method was developed and validated assuming a constant deuterium concentration of 0.0156% of the HCl concentration and incorporating HCl concentrations into the model. This approach also allowed small pressure and temperature fluctuations to be accounted for within the model.
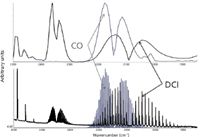
Figure 5: Process spectra containing DCl and some CO2 compared to reference spectra of CO at both 8-cm-1 and 1-cm-1 resolution.
Conclusions
Continuous at-line and online FT-IR implementation has proven to be effective for characterizing process streams that prove challenging from the perspective of traditional grab sampling and laboratory analysis. Care must be taken in sample handling and implementation to assure that a representative sample is delivered to the analyzer.
The value of FT-IR spectroscopy for process characterization is the information-rich nature of the data obtained and how that information is applied to optimize or troubleshoot the process. In many cases, a temporary online setup that provides a few weeks to a couple of months of stream data is all that is required for improvements to be chosen, implemented, and validated. However, in other cases the information collected by the temporary online FT-IR spectrometer indicates the need for a permanent online analysis to be used for closed-loop control or routine monitoring. In these situations, the stream composition data from the FT-IR analysis are used to specify the most robust, reliable, and economical solution for a permanent online analysis. In a few cases this might be a FT-IR system, but more commonly the permanent solution is an alternative spectroscopic-based solution, such as a filter photometer or tunable diode laser system. Use of full-spectrum FT-IR stream composition information while specifying a permanent analyzer system increases the success rate of the new application development and installations of process analysis technologies.
Serena Stephenson, Lamar Dewald, Esteban Baquero, Wendy Flory, Liane Mikolajczyk, and J.D. Tate are with The Dow Chemical Company. Direct correspondence to: SStephenson@dow.com
References
(1) K.R. Beebe, R.J. Pell, and M.B. Seasholtz, Chemometrics A Practical Guide (John Wiley & Sons, Inc., New York, 1998), Chapter 5.
(2) K.J. Clevett, Process Analyzer Technology (John Wiley & Sons, Inc. New York, 1986), Chapter 21.
(3) R.E. Sherman, Process Analyzer Sample-Conditioning System Technology (John Wiley & Sons, Inc, New York, 2002).
(4) P. Jaakkola, J.D. Tate, M. Paakkunainen, and P. Saarinen, Appl. Spectrosc. 51, 1159–1169 (1997).
(5) K. P. Huber and G. Herzberg, Molecular Spectra and Molecular Structure IV. Constants of Diatomic Molecules (Van Nostrand-Reinhold, New York, 1979).
AI Shakes Up Spectroscopy as New Tools Reveal the Secret Life of Molecules
April 14th 2025A leading-edge review led by researchers at Oak Ridge National Laboratory and MIT explores how artificial intelligence is revolutionizing the study of molecular vibrations and phonon dynamics. From infrared and Raman spectroscopy to neutron and X-ray scattering, AI is transforming how scientists interpret vibrational spectra and predict material behaviors.
Real-Time Battery Health Tracking Using Fiber-Optic Sensors
April 9th 2025A new study by researchers from Palo Alto Research Center (PARC, a Xerox Company) and LG Chem Power presents a novel method for real-time battery monitoring using embedded fiber-optic sensors. This approach enhances state-of-charge (SOC) and state-of-health (SOH) estimations, potentially improving the efficiency and lifespan of lithium-ion batteries in electric vehicles (xEVs).
New Study Provides Insights into Chiral Smectic Phases
March 31st 2025Researchers from the Institute of Nuclear Physics Polish Academy of Sciences have unveiled new insights into the molecular arrangement of the 7HH6 compound’s smectic phases using X-ray diffraction (XRD) and infrared (IR) spectroscopy.
