The Infrared Spectra of Polymers V: Epoxies
As we begin our survey of the infrared (IR) spectra of polymers containing C–O bonds, we discuss epoxies, perhaps the most economically important functional group in this category. Epoxy resins are widely used as adhesives, and you may have used them yourself to mend broken household items. In this column, the spectra of epoxides and the reaction via which they become useful is examined. The answer to last column’s Infrared Spectral Interpretation workshop will also be given.
As you probably noticed, the organization of this column series has been divided into sections based on specific chemical bonds. In the first five or so years of this column, there were multiple columns devoted to functional groups containing C-H, C-O, and C=O bonds. Now that we are five columns into our examination of polymer spectra, you may have noticed that these articles are structured similarly. The last four installments were devoted to polymers that contain C-H bonds. Here, we begin a series on polymers containing C-O bonds, and after that, we will discuss polymers with C=O bonds.
Compared to the polymer families containing C-H and C=O bonds, the family of C-O containing polymers is small for reasons I do not understand. Probably the most important polymers in this group are epoxies and epoxy resins, which are widely used as adhesives.
The Epoxy Group
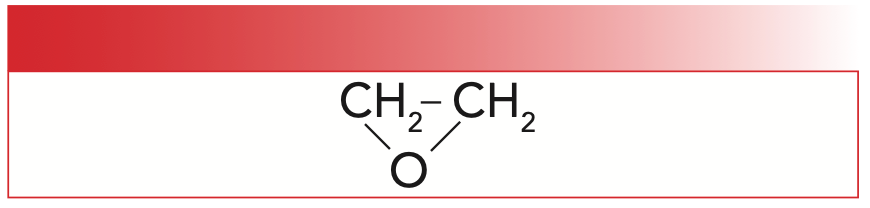
Epoxy resins are used so commonly that the word “epoxy” has entered the English language as a word that refers to a large group of glues and adhesives. We bandy about this word a lot, but what is an epoxy anyway? An epoxide is a molecule that contains an epoxy ring, which is a three-membered ring containing two carbons and one oxygen atom (1). The structure of the simplest epoxide, ethylene oxide, is seen in Figure 1.
FIGURE 1: The structure of ethylene oxide, a simple epoxide.
We know that carbon prefers to form single bonds with bond angles of approximately 109.5° (1). The bond angles in an epoxy ring are less than optimal, creating what is called ring strain, which means these rings are unstable and undergo ring opening reactions easily. Epoxides are monomers that react via ring opening reactions to form the sticky stuff we are familiar with.
Figure 1 shows that an epoxy ring contains two C-O single bonds. We have learned previously when we studied the spectra of alcohols and ethers that C-O stretching vibrations have strong peaks from 1300 to 1000 cm-1 (going forward all peak positions will be in cm-1 even if not noted) (2). However, epoxies are the first ring systems we have studied that have C-O bonds in them, and they follow our 1300–1000 rule. Figure 2 shows three stretching vibrations for the epoxide ring.
FIGURE 2: The ring stretching vibrations of the epoxide ring: S = Stretch, C = Contract.
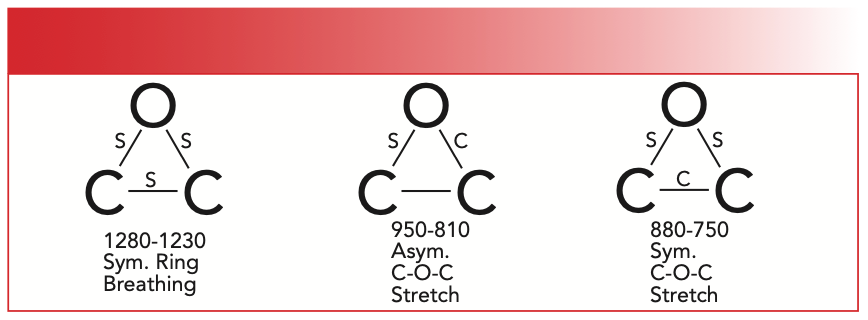
The S in Figure 2 means a bond stretches during that vibration, and a C means a bond contracts during a vibration. The left most vibration illustrated in Figure 2 is called the epoxide symmetric ring breathing vibration. As you can see, all three bonds in the ring stretch and contract in phase with each other, as though the ring were exhaling and inhaling. The peak for this vibration falls from 1280–1230, in the range where we normally see C-O stretching vibrations. The vibration illustrated in the center of Figure 2 is called the asymmetric C-O-C stretch, where one C-O bond stretches and the other contracts, similar to the asymmetric C-O-C stretch of ethers (3). The peak from the epoxide asymmetric C-O-C stretch appears from 950–810. The third vibration illustrated in Figure 2 is the symmetric C-O-C stretch, where the two C-O bonds stretch while the C-C bond contracts. The peak from this vibration is found from 880–750. The spectrum of an epoxide is seen in Figure 3.
FIGURE 3: The infrared (IR) spectrum of 1,2-epoxybutane. Peak A at 1261 is the symmetric ring breathing vibration, peak B at 904 is the asymmetric C-O-C stretch, and peak C at 831 is the symmetric C-O-C stretch.
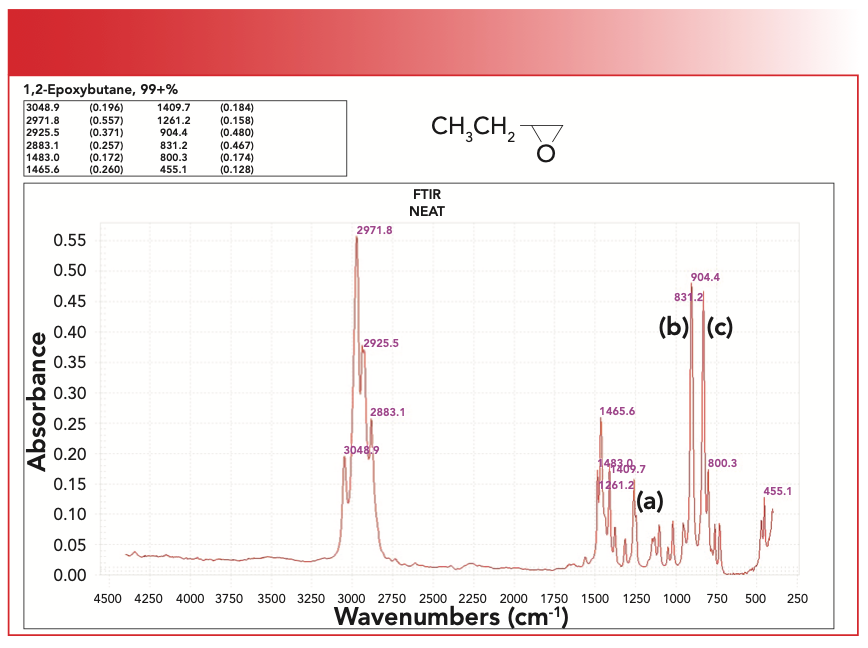
Peak A at 1261 is the symmetric ring breathing vibration. Note that this falls in the range where C-O stretching peaks traditionally fall. Peak B at 904 is the asymmetric C-O-C stretch, and peak C at 831 is the symmetric C-O-C stretch. Note how intense peaks B and C are, and that they fall at a relatively low wavenumber. Because the ring opening reaction of epoxides is part of how epoxy resins are made, monitoring the size of any of peaks A, B, or C can be used to determine the extent of cure of an epoxy resin.
When epoxy resins are made, they are typically reacted with a diamine to form a three-dimensional (3D) polymer network (Figure 4). A diamine is a molecule with two amine groups in it. If you have ever worked with epoxy resins, this is why they can be pretty smelly. We have already studied the spectra of amines (4,5).
FIGURE 4: The reaction of an epoxy ring with a diamine hardener to give an epoxy resin.
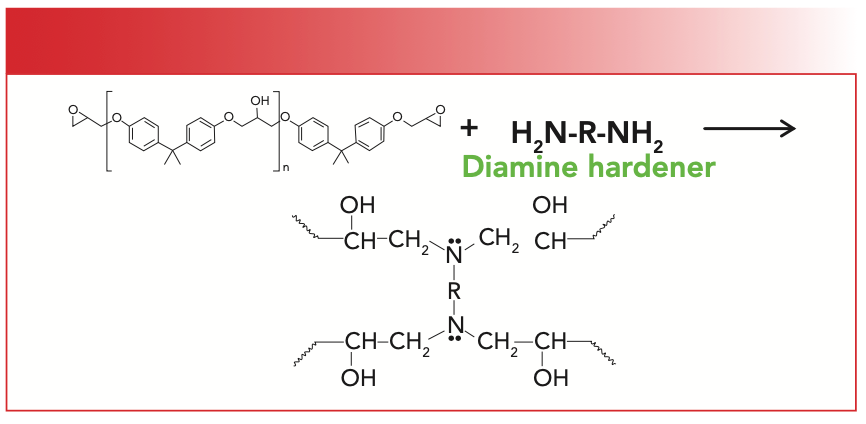
The epoxy ring reacts with the amine in a ring opening reaction that gives a 3D polymer network as illustrated in Figure 4. I would like to show you what the spectrum of this material looks like, but these materials are intractable and hard to analyze. For example, one could place an epoxide and diamine on an attenuated total reflectance (ATR) crystal and follow the course of the reaction that way (6). Once the reaction is over, the epoxy resin sticks to the crystal and cannot be removed, which means an ATR crystal is sacrificed.
Conclusions
Epoxides are molecules that contain a three-membered ring containing two oxygen atoms and one carbon atom. Their spectra exhibit a series of three peaks from the stretching and contracting of the bonds in this ring that fall from 1280–1230, 950–810, and 880–750. The last two of these are particularly intense. The size of these peaks can be used to follow the curing of an epoxy resin.

References
(1) A. Streitweiser and C. Heathcock, Introduction to Organic Chemistry (Macmillan, New York, NY, 1976).
(2) B.C. Smith, Spectroscopy 32(1), 14–21 (2017).
(3) B.C. Smith, Spectroscopy 32(5), 22–26 (2017).
(4) B.C. Smith, Spectroscopy 34(3), 22–25 (2019).
(5) B.C. Smith, Spectroscopy 34(5), 22–26 (2019).
(6) B.C. Smith, Fundamentals of Fourier Transform Infrared Spectroscopy (CRC Press, Boca Raton, FL, 2nd Ed., 2011).
(7) B.C. Smith, Spectroscopy 37(1), 8–12 (2022).
(8) B.C. Smith, Spectroscopy 30(4), 18–23 (2015).
Brian C. Smith, PhD, is the founder and CEO of Big Sur Scientific, a maker of portable mid-infrared cannabis analyzers. He has over 30 years experience as an industrial infrared spectroscopist, has published numerous peer-reviewed papers, and has written three books on spectroscopy. As a trainer, he has helped thousands of people around the world improve their infrared analyses. In addition to writing for Spectroscopy, Dr. Smith writes a regular column for its sister publication Cannabis Science and Technology and sits on its editorial board. He earned his PhD in physical chemistry from Dartmouth College. He can be reached at: SpectroscopyEdit@MMHGroup.com ●


Accurate Plastic Blend Analysis Using Mid-Infrared Spectroscopy
May 15th 2025Researchers at the Sinopec Research Institute have developed a novel method using virtually generated mid-infrared spectra to accurately quantify plastic blends, offering a faster, scalable solution for recycling and environmental monitoring.
How Spectroscopy and Science are Reshaping Gemology
May 13th 2025A historical and technical overview from the Gemological Institute of America (GIA) explores how advanced scientific instruments—particularly spectroscopic methods—have transformed gem identification. From refractometers to modern spectrophotometers, this deep dive highlights the evolving challenges and solutions in gem testing.
How Infrared Light Reveals the Truth About Gemstones
May 12th 2025New research from the Gemological Institute of America highlights the essential role of infrared spectroscopy in identifying gemstones, detecting treatments, and distinguishing natural from synthetic gems. The technique’s precision and non-destructive nature have made it an indispensable tool in modern gemology.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)