Wilhelm Conrad Röntgen and the Discovery of X-Rays
Volker Thomsen takes a look at the impact that the discovery of X-rays by Wilhelm Röntgen in 1895 has had on the world.

In 1895, Röntgen (Figure 1) was at the University of Wurzburg, Germany, experimenting with gaseous discharge tubes (Figure 2). When a high voltage is applied to the electrodes of a partially evacuated glass tube, a glow is observed. Back in 1838, Michael Faraday described the bright regions near the electrodes with a dark region in between (Faraday dark space). Later, physicist Johann Hittorf (1824–1914) observed glowing rays extending from the negative electrode to produce a fluorescent glow where they struck the glass walls. These were named "cathode rays" by Eugene Goldstein (1850–1931). Sir William Crookes (1832–1919) demonstrated that cathode rays could cast shadows of objects, turn a small wheel in their path, and be deflected by a magnet. In 1892, physicist Heinrich Hertz (1857–1894) showed that these rays could penetrate very thin metal foil. Röntgen, however, was to discover a different kind of ray.

Volker Thomsen
Röntgen was born on March 27, 1845, in Lennep, Prussia. His early schooling was in Holland. He received a diploma in mechanical engineering in 1868 from Zürich Polytechnic in Switzerland. One year later, he received his Ph.D. in the same subject for a thesis on the states of gases. He became an assistant to August Kundt, a professor of physics at Zürich, who awakened his interest in pure science. After a series of interim positions, he was appointed professor of physics at the University of Würzberg in 1889.

Figure 1
On the evening of November 8, 1895, Röntgen enclosed his discharge tube in a thick, black carton, excluding all light. Working in a dark room, he observed a plate coated with barium platinocyanide phosphor become fluorescent when placed in the path of the rays. The fluorescence persisted as far as 2 m from the discharge tube. In an attempt to record these rays, he switched to photographic plates. He found that different objects placed in the path of the rays showed varying transparency when recorded on a photographic plate. He demonstrated the effect of thickness on the transparency by building a step-wise wedge of tinfoil and photographing it in the path of the new rays.
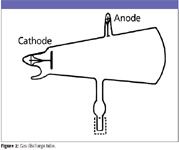
Figure 2
Röntgen continued to experiment throughout the night. In fact, it is said that he ate and slept in his laboratory for a time. At one point, he held his wife's hand in the path of the rays for a short time. When the photographic plate was developed, it showed an image of her hand. The bones and a ring she was wearing cast a distinct shadow. The flesh, being more permeable to the rays, cast a fainter shadow.

Figure 3
Röntgen showed that the new rays were produced by the impact of the cathode rays on material objects. He called them "X" rays because of their unknown nature. His first paper on the experiments, "On a New Kind of Ray, A Preliminary Communication," was submitted to the Wurzburg Physico-Medical Society on December 28, 1895, and was published before the end of the year (1). Very soon, many others were studying the new rays.
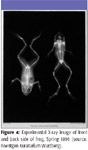
Figure 4
Later, asked what his thoughts were at the moment of discovering the new rays, he replied, "I didn't think, I experimented." Indeed, his experimentation was so thorough that one investigator (Silvanus Thompson) wryly observed that Röntgen had left "little for others to do beyond elaborating his work"(2).

Figure 5
It remained for physicist Max von Laue (1879–1960) and coworkers to show that X-rays have the same electromagnetic character as light, differing only in the higher frequency of vibration. (Von Laue won the Nobel Prize in physics in 1914 for discovery of the diffraction of X-rays by crystals.)
The applications of X-rays are ubiquitous today. Everyone has had a medical or dental X-ray. Industrial radiography is used for determination of defects in castings and weldments. X-ray fluorescence spectrometry is a key instrumental method for the determination of material chemical composition. X-ray diffraction is the most powerful tool for material structure determinations.
Sadly, the man who made all of this possible died penniless in 1923. He believed his discovery should be free to all and refused monetary gain.
Volker Thomsen, a physicist by training, has some 30 years of experience in elemental spectrochemical analysis (OES and XRF). He is currently a consultant in this area from his home in Atibaia, São Paulo, Brazil. His other interests include mineralogy and history of science. Occasionally, he still plays the blues harmonica. He can be reached at vbet1951@uol.com.br.
References
(1) W.C. Roentgen, "On a New Kind of Rays," Sitzungber. Phys.-Med. Ges. Wurzburg, 137 (1895). English translation online at www.emory.edu/X-RAYS/century_05.htm.
(2) The Health Physics Society, "Figures in Radiation History" http://www.orcbs.msu.Edu/radiation/radhistory/wilhelmrontgen.Html
(3) Roentgen Kuraturium Wurzberg. On the web at www.fh-wuerzburg.de/roentgen.
For further reading
Alexi Assmus, "The Early History of X Rays," Beam Line 25(2), 10–24 (Summer 1995). Available on the web at http://www.slacStanford.Edu/pubs/beamline/25/2/25-2-assmus.Pdf.
Otto Glasser, Wilhelm Conrad Röntgen and the Early History of the Roentgen Rays (Norman Publishing, Novato, California, 1993).

AI Shakes Up Spectroscopy as New Tools Reveal the Secret Life of Molecules
April 14th 2025A leading-edge review led by researchers at Oak Ridge National Laboratory and MIT explores how artificial intelligence is revolutionizing the study of molecular vibrations and phonon dynamics. From infrared and Raman spectroscopy to neutron and X-ray scattering, AI is transforming how scientists interpret vibrational spectra and predict material behaviors.
Advancing Corrosion Resistance in Additively Manufactured Titanium Alloys Through Heat Treatment
April 7th 2025Researchers have demonstrated that heat treatment significantly enhances the corrosion resistance of additively manufactured TC4 titanium alloy by transforming its microstructure, offering valuable insights for aerospace applications.