Analysis of Toxic Trace Metals in Pet Foods Using Cryogenic Grinding and Quantitation by ICP-MS, Part I
Part I of this two-part article focuses on the raw materials used in pet food manufacturing and all the potential sources of contamination.
The purpose of this study was to examine pet foods from a variety of sources to determine if potentially toxic elements were present in the foods. A range of " budget " to " premium " grade pet food samples were donated by pet owners or purchased in several different stores. The food samples were ground in a cryogenic freezer mill (dry samples) or blender (wet samples), digested with concentrated nitric acid, and analyzed for trace metal content by inductively coupled plasma-mass spectrometry (ICP-MS)Results were then compared to both Environmental Protection Agency Reference Dose (RfD) values and World Health Organization Tolerable Daily Intake (TDI) values, which are considered the daily oral exposure limits for the human population. Many of the pet foods sampled showed significant concentrations of various toxic metals. In many cases, the concentrations exceeded the extrapolated human limit values calculated to pet-size dosages. Part I of this article focuses on the raw materials that are used in pet food manufacturing processes and all the potential sources of contamination. Part II of the article will discuss how the data relate to the dietary exposure of toxic metals in those pet foods when calculated for the daily intake of an average-sized dog and cat. To get a better understanding of the impact on the health of the pets, the daily intake of toxic metals will compared to EPA and WHO human risk assessment guidelines.
P.Atkins, L.Ernyei, W.Driscoll, R.Obenauf, and R. Thomas
In the past decade, consumers have doubled their spending on pet-related products. According to the American Pet Products Association (APPA), in 2009, pet owners in the United States spent more than $17 billion dollars on pet food (1). Along with this increased spending, there has been increased controversy. The melamine pet food scare of 2007 affected millions of people and their pets as well as the pet food industry. The supplementation of protein sources with materials containing melamine and cyanuric acid killed hundreds of pets and sickened many others. The pet food scare highlighted the potential for contaminants and controversial ingredients that could be contained in pet food. In addition to organic chemical contaminants and additives, there is also the possibility of toxic elemental contamination from protein sources, fillers, and manufacturing processes. The search for healthy pet food therefore goes beyond the choice of a name-brand food or seemingly nutritious ingredients on a label.
According to the Pet Food Recall Product List on the U.S. Food and Drug Administration (FDA) website, there have been almost 1000 pet food recalls since 2006. These recalls have increased awareness of the inadequate testing of ingredients used in the pet food industry (2). Pet food is a multibillion dollar per year business controlled by four or five large corporations that produce 80% of U.S. pet food and a number of smaller, premium-brand pet food companies that produce the remaining 20%. Because the pet food industry is not as well regulated as is food for human consumption, the quality of many of the ingredients used for pet food often is considered to be inferior. The major claim of the manufacturers of premium brands is that of superior quality with superior ingredients. However, because regulations have not been defined as to the quality of pet foods, claims of superior quality ingredients are very subjective and not always supported by the information stated on the product label.
Pet Food Manufacture
The manufacture of pet food is a multistage process that exposes the ingredients to many potential contamination sources. Many of the same processes used to make food for human consumption are also used to make pet food. Dry pet food in the form of "kibble" accounts for more than 60% of all cat and dog food sales in the United States, whereas the most familiar form of wet pet food is sold in cans, trays, and pouches (3). The most common way to make dry pet food is extrusion. This process was adapted for making pet food in the 1950s based upon technology used to make breakfast cereals. Figure 1 illustrates the process of extrusion.
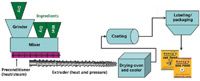
Figure 1: The manufacturing process of dry pet food (3).
To make an extruded dry pet food, wet and dry ingredients are brought together in a mixer to form a moist dough. The dough is heated in the preconditioner and then is introduced into a large grinder, where the primary cooking phase for dry extruded pet food products occurs. The dough is cooked under intense heat and pressure as it moves toward the open end of the extruder. The hot dough passes through a shaping die and knife where the small pieces expand rapidly into kibble. The food is dried in an oven until its moisture content is low enough to make it shelf stable, similar to a cookie or cracker. After cooling, dry food may pass through a machine that sprays on a coating, which is generally a flavor enhancer. The final result is a bag, box, or package of dry pet food or treats.
Pet food companies are required to follow the same federal regulations for making wet pet food products that human food companies must follow (4). The process is described in CFR Title 21, Part 113. Ingredients are incorporated into a mixer. Clean empty containers (cans, pouches, trays, and so forth) are filled to meet the weight advertised on the label. Sealed containers are cooked at a specified temperature to destroy any bacteria, viruses, or mold that could otherwise grow in the sealed container. After the containers have cooled, labels are applied to containers, resulting in the finished wet food products.
FDA Regulation of Pet Food
The FDA's regulation of pet food is contained in the Federal Food, Drug, and Cosmetic Act (FFDCA) and is similar to that for other animal feeds. According to the FFDCA, pet foods must be safe to eat, must be produced under sanitary conditions, must contain no harmful substances, must be truthfully labeled, and in the case of canned pet foods, must be processed free of microorganisms (5). However, there is no requirement that pet food products have premarket approval or testing. So even though the FDA is mandated to ensure that the ingredients have an appropriate function in the pet food, many ingredients such as meat products and grains are considered safe and do not require any additional permission for their use in the formula. Other substances such as minerals, vitamins, nutrients, flavorings, preservatives, fillers, binding agents, colorings, and processing aids may be generally recognized as safe (GRAS) for their intended use, but they must have approval as food additives (6).
Pet food labeling is also regulated at two levels. The current FDA regulations require proper identification of the product, net quantity statement, and name and place of business of the manufacturer or distributor. Labels also must include a proper listing of all the ingredients in the product in order from most to least, based on weight. Recent legislation in the Food and Drug Administration Amendments Act of 2007 requires the FDA to establish ingredient standards and definitions with respect to pet food; processing standards for pet food; and updated standards for the labeling of pet food that include nutritional and ingredient information. Additionally, the FDA also reviews specific marketing claims on pet food, such as "maintains healthy urinary tract," "reduces plaque," or "improves digestibility."
Ingredients and Additives in Pet Foods
As long as a pet food is considered safe to eat and contains no harmful substances, the FDA does not carry out or mandate any rigorous quality control testing of the products, particularly measuring the level of trace and heavy metals in the individual ingredients. Because most pet foods contain large numbers of ingredients, the likelihood that many of those ingredients could contain contaminants increases. There are quite a few ingredients common in pet foods that are ongoing points of controversy and debate. Some protein sources are questioned in regard to their source and quality. Many preservatives or chemical additives are questioned for safety and potential adverse health effects. Some of the more controversial ingredients and additives are listed below:
- Animal fat: Source of fat obtained from the tissues of mammals or poultry in the commercial processes of rendering or extracting. The animal source is not specified and is not required to originate from slaughtered animals.
- Animal meal: Source of protein, which is unspecified animal tissue that has been ground up.
- Animal digest: Source of protein, which is unspecified animal tissue that has been processed by chemical or enzymatic hydrolysis.
- Animal or meat by-products: Source of protein, which is the nonrendered, clean parts other than meat, derived from slaughtered mammals, including, but not limited to lung, spleen, kidneys, brain, liver, blood, bone, partially defatted low-temperature fatty tissue, and stomachs and intestines.
- Bone meal: Ground animal bones for calcium supplement.
- Fillers: Carbohydrates with very little nutritional value, including corn gluten, corn meal, wheat middlings, ground white rice, wheat germ, and soybean meal.
- Binding agents: Wheat gluten, a natural protein derived from wheat, is used as a thickener in the manufacture of some pet foods.
- Menadione: Synthetic vitamin K, which has been banned from human use in the European Union and the United States by the FDA but continues to be used in pet food.
- BHA (butylated hydroxyanisole) and BHT (butylated hydroxytoluene): These compounds are used as preservatives. They have been banned in the European Union due to health concerns but continue to be used in pet foods in the United States. Both compounds have been identified as possible human carcinogens and linked to tumors.
- Ethoxyquin: Preservative for seafood that has been suggested as a possible carcinogen.
- Propyl gallate: Antioxidant for cosmetics, products containing fats, and packaging that has been suggested as a possible carcinogen.
In addition to questions about these ingredients and additives, there is concern over the contaminants that may be present in pet food products. Trace-element levels of the animal parts used for the pet food will be impacted by the source of the feed or the nature of the soil and grass where the animals are grazing. Fish protein sources such as tuna are known to bioaccumulate high concentrations of heavy metals such as mercury. The grains and cereals that are used as fillers could contain contaminants from groundwater or airborne materials emitted by industrial processes or power plants. The geographical source of the ingredients could impact the quality control of the production process. The essential minerals and other additives may contain significant concentrations of trace metals. In addition to contaminants derived from the ingredients, the manufacturing process can pick up trace metals from the stainless steel equipment and containers used in the production process. In the case of canned cat and dog food, trace metals could be leaching out from the metal alloy used to produce the can.
The purpose of this study was therefore threefold: to measure the concentration of a wide array of elements present in both cat and dog foods and assess whether any of the potentially toxic elements were present in concentrations significant to a pet's health; to see if the metals' concentrations were reduced in foods that were sold as superior quality or were higher priced; and to determine if there was a difference in metal concentrations in foods obtained from metal cans or pouches compared to bagged or boxed dry food. To help in this evaluation, the pet's trace-metal exposure levels were measured based on its daily intake and then compared to EPA and WHO risk assessment guidelines calculated for an "average-sized" dog or cat.
Experimental
Reagents and Standards: Concentrated Optima-grade nitric acid was obtained from Fisher Scientific (Pittsburgh, Pennsylvania). Deionized water (ASTM I grade) was obtained from an in-house laboratory water filtration and processing system.
The SPEX CertiPrep standards (SPEX CertiPrep Group, Metuchen, New Jersey) used in this study included
- CLMS-2N: Claritas Multi Element Solution standard
- (10 mg/L: Ag, Al, As, Ba, Be, Bi, Ca, Cd, Co, Cr, Cs, Cu, Fe, Ga, In, K, Li, Mg, Mn, Na, Ni, Pb, Rb, Se, Sr, Tl, U, V, and Zn)
- CL-ICV-1: Claritas Initial Calibration Verification 1
- (1000 mg/L: Fe, K, Ca, Na, Mg, and Sr; 10 mg/L: Ag, Al, As, Ba, Be, Cd, Co, Cr, Cu, Mn, Mo, Ni, Pb, Sb, Se, Tl, V, Zn, Th, and U)
- CLU2-2Y: Claritas Uranium Single Element standard (1000 mg/L)
- CLHG2-1AY: Claritas Mercury Single Element standard (10 mg/L)
Standards were diluted to microgram-per-kilogram concentrations appropriate for inductively coupled plasma–mass spectrometry (ICP-MS) analysis in 5% nitric acid in deionized water solution to match the acid matrix of samples. Standards of 0.1, 0.4, 1.0, 4.0, and 10 µg/kg were made up in 5% nitric acid, and matrix matched for ICP-MS calibration.
Blank Preparations: Optima-grade nitric acid used in digestion was added to the deionized water blanks before analysis in a concentration comparable to that of the acid concentration found in the pet food samples. In addition to water blanks, Optima-grade nitric acid was processed as a sample in the microwave vessel to determine contamination from the PFTE vessels or any memory effects of the previous samples. After each pet food sample digestion run, 10 mL of nitric acid was discarded from the microwave vessels. A second 10-mL nitric acid digestion was then performed and the digest was diluted in the same method as the pet food samples. These vessel blanks were analyzed before the corresponding samples and the blank results were subtracted from the immediately subsequent sample analysis.
Sample Collection and Preparation: For this investigation 58 cat and dog foods were bought from local stores or donated by the authors and other pet owners. The samples consisted of 31 dry food and 27 wet food varieties. Of the 31 dry foods, 18 were dog food and 13 were cat food samples. The wet foods comprised 13 dog food and 14 cat food samples, representing pet food contained in cans and pouches. The wet dog food cans and pouches ranged in size from large 375-g cans to small 175-g cans and pouches. The cat food was also packaged in cans and pouches and ranged from a large can averaging 175 g to small cans averaging 75 g. The range of quality of the pet foods was from "discount" to "gourmet" brands. Pet food prices ranged from the "bargain" store foods priced at $0.02/oz to gourmet or specialty foods purchased from pet suppliers priced at $0.42/oz. Three canned foods for human consumption were tested, including tuna fish, sardines, and chicken, which were sampled for comparison and control purposes. The three proteins intended for human consumption were packed in water in metal cans. The size of the packaging for the human food proteins was 3–4 oz (85–115 g).
Dry Food Preparation: Dry cat and dog food samples (12–16 g) were ground to a uniform powder in a cryogenic mill (SPEX SamplePrep 6870 FreezerMill). Cryogenic grinders are laboratory mills that chill samples in liquid nitrogen and pulverize them into a fine powder. The design incorporates an insulated tub into which liquid nitrogen is poured. The grinding mechanism is a magnetic coil assembly suspended in the liquid nitrogen bath (7). Cooling materials to temperatures approaching –200 °C makes flexible samples extremely brittle so they can be pulverized quickly by impact milling. This ability allows difficult-to-process samples (bone, rocks, polymers, metals, and food materials) to be processed more efficiently before analysis.
Each dry pet food sample was placed in a closed grinding vial and thoroughly cooled before it was ground by the magnetic coil shuttling the impactor rapidly back and forth, which pulverized the sample against the end plugs of the vial (Figure 2). The type of vial and impactor for grinding samples was selected to reduce any potential cross contamination of metals. In this study, polymer vials and polymer-encased impactors were selected instead of metal vials and impactors to reduce metal contamination.
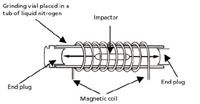
Figure 2: Schematic of the cryogenic freezer mill used in this study.
Wet Food Preparation: Canned food was processed in a tabletop blender to a homogenized paste consistency. Deionized water was added to samples as needed to facilitate blending. Water added to samples was measured and recorded. The weight of the added water was subtracted from the sample weight at analysis.
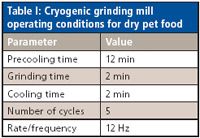
Table I: Cryogenic grinding mill operating conditions for dry pet food
Sample Digestion: A 1-g amount of the powdered or blended sample was digested with 10 mL of nitric acid in a MARS 5 microwave oven (CEM, Matthews, North Carolina). The digestion program can be found in Table II. After digestion, samples were diluted to a 50-mL volume with deionized water. Before analysis, samples were diluted 1000-fold using deionized water.
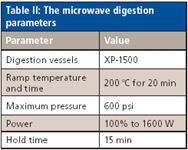
Table II: The microwave digestion parameters
Instrument Conditions: Primary sample analysis was conducted using an Optima 7300 ICP optical emission spectrometry (OES) system (PerkinElmer LAS, Shelton, Connecticut). The ICP-OES system was used to determine the macroelemental composition of the foods to select standards that would allow for proper matrix interference evaluation. Elements measured by ICP-OES included aluminum, calcium, copper, iron, magnesium, manganese, phosphorus, potassium, selenium, silicon, sodium, sulfur, and zinc.
All trace-element determinations were carried out using ELAN 6000 and ELAN 6100 DRC ICP mass spectrometers (PerkinElmer LAS). The ICP-MS operating conditions are shown in Table III. It can be seen that multiple isotopes were used for many of the elements to evaluate the spectral complexity of the samples and also for mathematical equation correction purposes. Wherever possible, the most sensitive isotope free of spectral interferences was chosen for the quantitation. It should also be noted that 75 As, 52 Cr, and 51 V are known to have severe polyatomic spectral interferences from 40 Ar35 Cl, 40 Ar12 C, and 35 Cl16 O respectively, which are produced by interaction of the plasma gas ions with the sample matrix and solvent. For this reason, the dynamic reaction cell (Elan 6100 DRC) utilizing ammonia gas was used to reduce these interferences (8).

Table III: ICP-MS system standard operating conditions used for a suite of trace elements in pet food samples.
Results and Discussion
The results of a select group of 15 potentially harmful or toxic elements categorized by pet (dog or cat) and type of food (dry or wet cans or pouches) are shown in Tables IV–VII. Tables IV and V represent dry and wet dog food, respectively, and Tables VI and VII show dry and wet cat food, respectively. The human canned food, including tuna fish, sardines, and chicken, is shown in Table VIII. All the results are expressed in units of micrograms per kilogram. The 15 elements selected were chosen due to either their toxic significance or their potential or perceived toxicity.
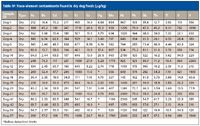
Table IV: Trace-element contaminants found in dry dog foods (µg/kg)
The analysis of all the pet food samples showed that the highest concentrations of toxic elements were found in the dry foods of both cats and dogs. Out of the elements studied, dry food had the highest elemental content for 13 of the 15 elements examined. Dog food had the highest result for nine of the 15 toxic elements and cat food had the highest concentration for six of the 15 elements.

Table V: Trace-element contaminants found in canned or pouched wet dog foods (µg/kg)
The dry dog food contained the highest concentrations of the following elements: beryllium, cadmium, cesium, antimony, thorium, thallium, uranium, and vanadium. Several of these potentially toxic elements were detected at concentrations near or above 1 mg/kg. Four dry dog foods had cobalt concentrations over 500 µg/kg (Dog-5, -19, -41, and -57). Dog-5 and -19 had the highest cobalt concentrations over 800 µg/kg. Significant amounts of nickel were found in a large percentage of the dry dog foods, with the highest concentrations reaching almost 3 mg/kg (Dog-10). Lead concentrations for all the dry dog food samples were generally less than 200 µg/kg, but one dry dog food contained just under 1 mg/kg (Dog-42). Generally, less than 100 µg/kg of antimony was detected in the dry dog samples, except for two samples found to contain over 500 µg/kg including Dog-12, which contained almost 1 mg/kg of antimony. Seven dry dog foods were found to have over 100 µg/kg of uranium with four of the seven containing more than 500 µg/kg of uranium. The highest uranium level detected of all the pet foods was 864 µg/kg in the Dog-11 sample. Vanadium was found at concentrations over 1 mg/kg in four of the 18 dry foods tested. The highest level of vanadium was 2.4 mg/kg in the Dog-11 sample.

Table VI: Trace-element contaminants found in a number of different dry cat foods (µg/kg)
The wet dog foods contained lower concentrations of the toxic elements studied than the dry dog foods. The majority of the wet dog food samples had concentrations of the selected elements at or below 500 µg/kg. Only nickel and chromium, out of the 15 elements studied, were found at concentrations at or over 1 mg/kg. It is important to note that all wet pet foods were tested as served and were not calculated to dry weight equivalents. The study focused on the amount of potential contamination exposure to the average pet through daily consumption, not dry weight contamination concentrations.

Table VII: Trace-element contaminants found in a number of different canned or pouched wet cat foods (µg/kg)
The dry cat foods contained the highest results for five of the 15 elements including arsenic, cobalt, molybdenum, nickel, and lead. The Cat-43 sample contained the highest concentration of arsenic at nearly 200 µg/kg. The concentrations of arsenic in the dry cat foods were almost all in the range of 100 µg/kg or over. Five potentially toxic elements were detected at concentrations around or over 1 mg/kg. The majority of wet cat food samples had concentrations over 500 µg/kg for chromium, molybdenum, and nickel. A total of 12 out of the 13 cat food samples had concentrations of molybdenum greater than 500 µg/kg. Five of the 13 samples had greater than 1 mg/kg of molybdenum. The highest molybdenum reading for all the pet food samples was 2.3 mg/kg in the dry cat food sample Cat-7. Chromium concentrations were also consistently above 500 µg/kg for the majority of the dry cat food samples with the highest dry cat food level at 1.2 mg/kg of Cr. All the dry cat food samples contained greater than 500 µg/kg of nickel. Eight of the 13 dry cat food samples contained over 1 mg/kg of nickel, and four of these samples contained over 2 mg/kg of Ni (Cat-40, -46, -47, and -49). The highest lead level of all the pet foods tested was found in the Cat-14 sample, which contained almost 6 mg/kg of lead.

Table VIII: Trace-element contaminants found in three canned foods made for human consumption
The wet cat foods showed the overall lowest concentrations of the toxic elements studied than any of the other pet foods studied. Only one wet cat food had a result of over 1 mg/kg concentration of a potentially toxic element. The Cat-25 sample contained 2.8 mg/kg of nickel and the Cat-37 sample contained just less than 1 mg/kg of nickel.
Three canned foods for human consumption were selected to compare with the pet foods. The comparison of the human foods to the wet and dry pet foods showed that there were relatively low concentrations of all the potentially toxic compounds. The concentrations of all the elements except nickel were found to be below 100 µg/kg concentrations. Nickel concentrations in the seafood were under 1 mg/kg. The canned chicken contained 948 µg/kg of nickel. The sardine samples contained the highest level of arsenic of the three human foods at 30 µg/kg. The tuna sample contained the highest amounts of mercury of any of the human foods (53 µg/kg).
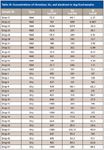
Table IX: Concentrations of chromium, tin, and aluminum in dog food samples
Packaging Contamination Elements: One of the objectives of this study was to identify if the packaging materials, such as the metal cans, were responsible for higher concentrations of metals in the pet food. Modern food cans generally are made of some form of tin-coated steel or aluminum. In many cases, the interiors of the cans are lined with epoxy, resins, bisphenol A, or other materials to reduce metal leaching. The pet food samples were examined for concentrations of aluminum, chromium, and tin. The resulting concentrations of these three metals did not show any correlation between the packaging type and increased concentrations of aluminum, chromium, or tin. Tables IX and X show the concentrations of chromium, tin, and aluminum found in wet versus dry foods in the dog and cat food samples. The overall concentrations of these three metals were found to be higher in the dry food samples than in the wet food samples. The highest level of tin found in all the pet food samples (9.4 mg/kg) was in dry dog food (Dog-12). It appears that in the case of the wet food samples studied, that the metal cans did not contribute significantly to the concentrations of chromium, tin, or aluminum when compared to the dry food samples.
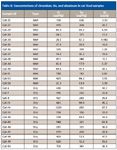
Table X: Concentrations of chromium, tin, and aluminum in cat food samples
The presence of several other elements in some of the pet food samples was unexpected. Uranium, beryllium, and thorium are often associated with nuclear energy and mining. As stated earlier, concentrations of over 500 µg/kg of uranium were found in several of the dry dog food samples. A few of the dry cat food samples had concentrations of over 200 µg/kg of uranium. In these samples of high uranium concentrations, there were also found to be the highest concentrations of both beryllium and thorium. The majority of the pet food samples had concentrations of beryllium and thorium below 10 mg/kg. In the pet foods in which concentrations of uranium exceeded 200 µg/kg, the concentrations of both beryllium and thorium were above 9 µg/kg.
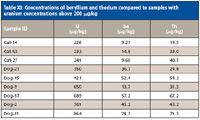
Table XI: Concentrations of beryllium and thorium compared to samples with uranium concentrations above 200 µg/kg
Part II of this article will examine in detail the data shown in Tables IV–XI and will calculate the toxic metal exposure levels of the pets on a daily basis, based on typical size portions. It also will look for a correlation with the cost of the individual pet foods. The exposure levels will then be compared with EPA and WHO risk assessment values generated for the human population, scaled to the weight of a medium-sized dog or an average-sized cat.
P. Atkins is Product Applications Specialist, L. Ernyei is Senior ICP Spectroscopist, W. Driscoll is Senior ICP-MS Spectroscopist, and R. Obenauf is President, all at SPEX CertiPrep. R. Thomas is Principal Consultant with Scientific Writing Solutions. The authors can be contacted at crmsales@spexcsp.com.
References
(1) American Pet Products Association (APPA) website: http://www.americanpetproducts.org
(2) Pet Food Recall Product List, U.S. Food and Drug Administration: http://www.accessdata.fda.gov/scripts/newpetfoodrecalls
(3) "How Pet Food is Made," Pet Food Institute: http://www.petfoodinstitute.org/Index.cfm?Page=DryPetFood
(4) Thermally Processed Low Acid Foods Packaged in Hermetically Sealed Containers, Code of Federal Regulations (CFR) Title 21, Part 113, U.S. Food and Drug Administration: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm
(5) The Federal Food, Drug, and Cosmetic Act (FFDCA), U.S. Food and Drug Administration: http://www.fda.gov/AnimalVeterinary/Products/AnimalFoodFeeds/PetFood/UCM2006475
(6) Regulation of Pet Food, U.S. Food and Drug Administration: http://www.fda.gov/AnimalVeterinary/ResourcesforYou/ucm047111.htm
(7) Product Specifications for 6770 FreezerMill, SPEX SamplePrep, Metuchen, New Jersey: http://www.spexsampleprep.com/freezermill
(8) S.D. Tanner and V.I. Baranov, Atomic Spectroscopy 20(2), 45–52 (1999).

Trending on Spectroscopy: The Top Content of 2024
December 30th 2024In 2024, we launched multiple content series, covered major conferences, presented two awards, and continued our monthly Analytically Speaking episodes. Below, you'll find a selection of the most popular content from Spectroscopy over the past year.
Best of the Week: Hyperspectral Imaging, ICP-MS Analysis of Geological Samples, Product Roundup
October 18th 2024Top articles published this week include an article about hyperspectral imaging in human skin research, a peer-reviewed article about analyzing geological samples using atomic spectroscopy techniques, and an equipment roundup piece about the latest products in the industry.
Analyzing Mercury Isotopes: A SciX Interview with Frank Vanhaecke, Part 2
October 17th 2024In this preview to the upcoming SciX 2024 conference next week, Spectroscopy sat down with Frank Vanhaecke to discuss about his educational background and what he is looking forward to about the upcoming conference.