Underwater Mass Spectrometry
This month's column describes the use of mass spectrometers in submarines and their more recent use for direct sampling of compounds dissolved in water at ever-increasing depths.

Mass spectrometers have been located "under the sea" longer than most folks realize, providing some of the first examples of portable instrumentation. Mass spectrometers installed on submarines monitor air quality; Wyatt (1) provides an overview of the development of these instruments for the U.S. Navy, describing unique instrumentation dating back to 1972. Interestingly, the first submarine prototypes drew from the design of the mass spectrometry (MS) module that flew on Skylab. In the submarine environment, monitoring of carbon dioxide in the air (which many guess was the primary use of the mass spectrometer) was initially accomplished by other means and the mass spectrometer was instead used to monitor gases derived from solvents and refrigerants, as well as the volatile gases from various specialized components found exclusively on submarines. These gases could become toxic or could react with each other to form secondary contaminants in an enclosed environment filled with a great deal of heat-producing machinery. It is worth noting that the U.S. Navy only recently banned smoking on submarines; therefore, the boat atmosphere was reasonably expected to be filled with a variety of compounds, some of which may have been innocuous, but many of which required constant monitoring. The robustness of these early MS instruments was clearly a primary concern. There was also a strong need to avoid false positive conclusions that would require actions that might compromise the safety of the boat and its crew. In his article, Wyatt states that the on-board instrument was sited adjacent to a hatch through which supplies were delivered. The instrument therefore had to withstand the occasional deluge of salt water as well as dirt related to the in-and-out flux of men and supplies. Because the cost of a mass spectrometer is but a small fraction of the cost of a submarine, the research, development, and testing was expanded to include almost every conceivable instrument type for on-board installation. The basic analytical need for air monitoring aboard submarines continues, and smaller instruments based on new technology and different mass analyzers continue to be developed for this application (2,3).
Operation of a mass spectrometer on a submarine, despite the specialized specifications, could still depend on reliable power and a fixed relative location. The instrument was movable (because the submarine moved) but not subject to the same constraints as a truly portable mass spectrometer. Furthermore, despite the location of the mass spectrometer, an instrument in Skylab or aboard a submarine still operates within the human life support range of temperature and pressure. Mass spectrometers that transit interplanetary space or land on the surfaces of other planets operate in a totally different environment. The design of these voyaging instruments reflects different and stringent constraints of power load, pressure management, ionization, mass analysis, data collection and encoding, and transmission of the data to mission control.
Let's now consider MS instruments that sample from water, which may be sited in yet another physical niche, depending on the sample collection process. The water column contains dissolved gases and soluble compounds (both natural and pollutants). In addition to concentrations of such targeted compounds measured at a given depth, the vertical and horizontal variation in the concentrations might also be measured to delineate current- or temperature-driven transport processes, adding a time dimension to the measurement. Finally, certain specialized submarine environments such as thermal vents or submerged wrecks are scouted with submersibles equipped with MS (and other) analyzers, and a three-dimensional picture of plume components, some of which might be unexpected, potentially can be established.
How then do we sample compounds directly from water? We can "grab" a sample at a specified location and transport it to the mass spectrometer for analysis. The MS instrument could be on land, of course, but transport to the instrument will then take time and the sample may change in the interim. The MS instrument also can be on-board a ship for close to real-time measurements and relaxed sample storage and transport requirements; this setup enables possible real-time decisions about where and what to sample. Ultimately, we can place the mass spectrometer itself in the water. In such a true underwater instrument, the instrument design clearly is going to be markedly different from instruments on boats or ships, and most of the differences will deal with the requisite attainment of a vacuum in the mass analyzer.
We focus first on the sampling process in underwater mass spectrometers. Volatile species dissolved in water can be sampled by heating the sample to force volatilization or by bubbling gas through it, and then directing the gas stream to a mass spectrometer. Direct sampling from the liquid often uses a different approach, and we have a great deal of experience in this area. Process-control mass spectrometry often involves sampling directly from liquid streams tapped at some point in a production process (4). A common device used for sampling is a membrane introduction system; the resulting analytical approach is known as membrane introduction mass spectrometry (MIMS), a technique that is now about 20 years old in the modern area of application (5). The membrane is usually composed of polydimethylsiloxane (PDMS) in any of several physical configurations that allow liquid to flow to and away from the membrane. A valve system is used to alternate between the liquid sample and reference (in this case background water) samples. The transport of samples through the membrane (sample permeability) is dependent on pressure, temperature, and hydrodynamic (flow rate) factors. The membrane in most underwater sampling systems is heated to a constant temperature, and more sophisticated systems maintain a steady flow of sample across the membrane. But pressure will, of course, vary with sampling depth. Calibration of the membrane permeability is therefore a matter of ongoing concern and is described thoroughly by Bell and colleagues (6) using a semiempirical mathematical fit to reference data. The fit values were determined for dissolved gases such as methane, oxygen, and hydrogen sulfide. The organic compounds of interest in this calibration study were benzene, chloroform, 1,4-dioxane, and toluene. It is worth noting that MIMS systems are usable to depths of at least 2000 m, which is truly a remote location for sampling and a very remote location for instrument maintenance. Laboratory calibrations show that the PDMS membrane permeability exhibited hysteresis, which was thought to be due to changes in the membrane elasticity. These results suggest that membrane aging may become a significant issue in a deployed array of MIMS-based remote instruments.
Short and colleagues (7) described two underwater MS instruments in detail and identified the primary challenge in instrument design as the maintenance of the vacuum for the mass analyzer. Sampling through the membrane invariably places a gas load on the pumping system. A throughput pumping system removes molecules from inside the mass spectrometer to the outside, but in the case of an underwater mass spectrometer the "outside" is limited by being underwater. Entrainment and trapping pumping systems (such as ion pumps or sieve pumps) do not exhaust into the external environment, but they may have more limited pumping capacity. Given the constraints inherent on the pumping system, reasonable compromises must be made. Short and colleagues describe two systems capable of sustained operation for as long as 12 days at depths as low as 30 m. Figure 1 shows the system configured with a quadrupole mass filter. The diaphragm pump serve as the fore pump for the small turbomolecular pump that maintains the mass analyzer pressure at 10-5 Torr or less. The exhaust of the diaphragm pump is routed to a separate pressure chamber (not shown). The instrument is designed as a modular unit, so parts can be swapped as needed. For instance, the quadrupole mass analyzer can be replaced with an ion trap modified from a commercial unit. Both instruments use an electron ionization source. The entire unit as shown in Figure 1 is 1.4 m long, consumes about 95 W of power during operation (drawn from lead-acid battery packs), can be operated through a radio frequency link, and is neutrally buoyant in water, although it weighs 39 kg in the laboratory. This latter characteristic eases placement of the instrument at the specified depth. The unit was deployed on a submersible vehicle and also was towed behind a surface boat. In a proof-of-concept demonstration, the presence of a nearby gasoline-driven boat was correlated with an increase in the signal for m/z 78 and m/z 91 (benzene and alkylated benzenes, respectively) in the mass spectrum.
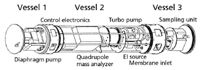
Figure 1: Schematic of a self-contained underwater mass spectrometer using a membrane introduction system and a quadrupole mass filter. Adapted from reference 7.
Later work by the same group (8) positioned this underwater MS instrument near a geothermal vent at a depth of 17.5 m. The analysis showed that the warmer vent water was depleted in oxygen and enriched in carbon dioxide relative to the concentrations of these gases in the surrounding water. This was a single-point measurement; a set of three-dimensional measurements would track this change in dissolved gas concentration around the vent. In another field deployment, the instrument (with an ion-trap mass analyzer) was set in stationary position within a marina and operated continuously for 72 h to monitor levels of hydrocarbon pollution. The data was displayed in a three-dimensional array (two dimensions for location and one dimension for concentration) to plot the distribution of dimethyl sulfide in a controlled-release experiment.Full-scan data were recorded every 5 s and transmitted through a wireless Ethernet link to the data storage computer, which also logged the GPS coordinates of the instrument and sample over a 1-h sampling period. A visually tracked dye was released at the same time as the dimethyl sulfide. The visual distribution of the dye was compared to the MS-generated distribution and indicated that hysteresis or delayed sampling effects due to the permeability of the PDMS membrane may have broadened the measured distribution. This conclusion assumes that the dye and the dimethyl sulfide are transported at similar rates through the water. The dependence of the quality of the measurement on the validity of the sampling protocol holds true for underwater MS as it does for any other use of mass spectrometry. Although MIMS is mature within the application area of process control, substantial additional development will be needed for quantitative and precise measurements in underwater MS.
McMurty and colleagues (9) listed an ambitious set of goals for an underwater MS system, including six months of autonomous operation at depths as low as 4000 m. The proposed instrument is similar in size, design, and power consumption to packages previously described (7,8). To conserve power and maintain extended operation, there are two modes of operation envisioned. The first is a "sleep" mode, with measurements taken at a prearranged interval. The second is a related variant that has a long sampling interval (for example, lasting 12 h), which is required because of the low permeability of compounds of interest (hydrocarbons) through the membrane at high pressures and cold temperatures. In either mode, the maintenance of the vacuum within the mass spectrometer will be extremely difficult over an extended period of time. Calibration of the instrument response will also be a significant analytical challenge because there are no comparative measures.
The deep ocean is in many aspects a deep analytical mystery, and therein resides the alluring challenge in designing instruments of exploration. Readers of this column over the past 15 years are well-versed in the fundamental aspects of MS instrumentation. The need for vacuum in the mass analyzer of the MS instrument is not subject to compromise. The instrument, its electronics, and the sampling system can be miniaturized and made portable. As of now, the vacuum requirements can be met only with plenums and chambers of some substantial size. The buoyancy of such reservoirs underwater is an engineering issue, but not one with which designers are unfamiliar. We build submarines, after all. If there is a need for substantial size in a pumping plenum, then the ability to build small mass spectrometers is almost secondary. One logical outcome would be to distribute the analyzers and centralize the vacuum. The advantages of duplicate measurements or measurements within different sampling time windows (perhaps with concentration of analytes) is clear. Additionally, there is no requisite need to design an underwater mass spectrometer, with a single multipurpose mass analyzer, to scan a large mass range and cover dissolved gases of low molecular mass in the mass spectrum and higher mass ions indicative of pollutants such as hydrocarbons. The concept of multiplexed mass spectrometers connected to a common vacuum reservoir and controlled by a common semiautonomous heuristic computer is a foreseeable direction of development. Another direction would be to design mass spectrometers that are a factor of 10 smaller (easing the vacuum requirements), perhaps even with a prepumped vacuum chamber. Such micro-MS instruments would be operational for only a short time, after which they would have to be retrieved and reconditioned. The ultimate goal of an array of MS-based sensors in the oceans is not so far afield conceptually from arrays of sensors already deployed on the ocean floor and in permanent floating buoys. Thinking "outside the box" must include getting your analytical feet wet "under the sea."
References
(1) J.R. Wyatt, Undersea Warfare, the Official Magazine of the U.S. Submarine Force 3(2), (2001). See: http://www.globalsecurity.org/military/library/report/2001/breathe.htm.
(2) W. Niu, G. Stewart, L. Davidson, T.L. Shadle, and A. Davis, Proceedings of the International Conference on Environmental Systems (Colorado Springs, Colorado) (SAE International, 2004).
(3) S. Hunt and R.F. Tindall, Int. J. Mass Spectrom. Ion Processes 80, 277–287 (1984).
(4) J. Workman, Jr., K.E. Creasy, S. Doherty, L. Bond, M. Koch, A. Ullman, and D.J. Veltkamp, Anal. Chem. 73(12), 2705–2718 (2001).
(5) R.C. Johnson, R.G. Cooks, T.M. Allen, M.E. Cisper, and P.H. Hemberger, Mass Spectrom. Rev. 19, 1–37 (2000).
(6) R.J. Bell, R.T. Short, F.H.W. Van Amerom, and R.H. Byrne, Environ. Sci. Technol. 41(23), 8123–8128 (2007).
(7) R.T. Short, D.P. Fries, M.L. Kerr, C.E. Lembke, S.K. Toler, P.G. Wenner, and R.H. Byrne, J. Am. Soc. Mass Spectrom., 12, 676–682 (2001).
(8) G.P.G. Kibelka, R.T. Shirt, S.K. Toler, J.E. Edkins, and R.H. Byrne, Talanta 64, 961–969 (2004).
(9) G.M. McMurty, J.C. Wiltshire, and A. Bossuyt, Oceans- Europe 2005, 395–400 (2005).

Kenneth L. Busch Kenneth L. Busch has been on a submarine, but not while it was underway. He has been through Skylab, too, but only the Skylab display at the Air and Space Museum in Washington, D.C. He came close to a submersible mass spectrometer once when the cooling water line for the diffusion pump ruptured. After we deploy arrays of sensitive and specific MS-based detectors in the oceans, we may develop an understanding of ocean chemistry and its role in the Earth ecosystem, integrating massive amounts of data in a complex computational model, much like we do now in meteorology and weather forecasting. This column is the sole responsibility of the author, who can be reached at WyvernAssoc@yahoo.com

High-Speed Laser MS for Precise, Prep-Free Environmental Particle Tracking
April 21st 2025Scientists at Oak Ridge National Laboratory have demonstrated that a fast, laser-based mass spectrometry method—LA-ICP-TOF-MS—can accurately detect and identify airborne environmental particles, including toxic metal particles like ruthenium, without the need for complex sample preparation. The work offers a breakthrough in rapid, high-resolution analysis of environmental pollutants.
The Fundamental Role of Advanced Hyphenated Techniques in Lithium-Ion Battery Research
December 4th 2024Spectroscopy spoke with Uwe Karst, a full professor at the University of Münster in the Institute of Inorganic and Analytical Chemistry, to discuss his research on hyphenated analytical techniques in battery research.
Mass Spectrometry for Forensic Analysis: An Interview with Glen Jackson
November 27th 2024As part of “The Future of Forensic Analysis” content series, Spectroscopy sat down with Glen P. Jackson of West Virginia University to talk about the historical development of mass spectrometry in forensic analysis.
Detecting Cancer Biomarkers in Canines: An Interview with Landulfo Silveira Jr.
November 5th 2024Spectroscopy sat down with Landulfo Silveira Jr. of Universidade Anhembi Morumbi-UAM and Center for Innovation, Technology and Education-CITÉ (São Paulo, Brazil) to talk about his team’s latest research using Raman spectroscopy to detect biomarkers of cancer in canine sera.