Calibrationless Semiquantitative Analysis of a Heterogeneous Sample Using Raman Microscope Mapping
Advances in Raman spectroscopy and imaging generate large amounts of information pertaining to the chemical and physical composition of materials. The distillation of meaningful and useful information from such quantities of data can be challenging. New image analysis software combined with powerful chemometric techniques permit an analyst to perform rapid calibrationless and quantitative analysis and discover features easily overlooked using less rigorous methods. This article describes mapping and analysis of a painkiller tablet using a dispersive Raman microscope and accompanying software.

Multivariate curve resolution identified four components, including one present as a minor impurity. Binary image maps generated from component concentration maps were used to calculate the relative concentrations of the four components. The impurity was identified from a library search to be titanium dioxide. The probability of finding and identifying a minor impurity by trial-and-error component identification is low.
Microscopy is used frequently to assess the microheterogeneity and to identify components in solid samples. One common application in the pharmaceutical industry is the mapping and quantification of excipients and active pharmaceutical ingredients in tablets. Both Fourier transform-infrared (FT-IR) and Raman microscopy have proven useful for this application. Raman microscopy is particularly valuable for this application, because Raman spectroscopy can identify not only the molecular species present, but also can distinguish between crystal polymorphs and amorphous forms. In addition, unlike FT-IR imaging microscopy, Raman spectra are not subject to artifacts generated by differences in the sample surface morphology. Dispersive Raman microscopes use excitation lasers in the visible wavelength range. This results in excellent spatial resolution down to 1 μm.
New imaging software now incorporates sophisticated chemometric and image analysis tools. When combined with library search capabilities, dispersive Raman tablet mapping using this software can identify the tablet components and perform a calibrationless semiquantitative analysis of their distribution.
Experimental
The Thermo Nicolet Almega XR dispersive Raman microscope, together with OMNIC Atlμs mapping software, was used (Thermo Electron, Waltham, Massachusetts).
The workflow for calibrationless quantitative analysis of a heterogeneous tablet sample using Raman microscopy consists of the following steps:
- Map the area of interest by collecting Raman spectra using the Almega XR Raman dispersive microscope.
- Perform multivariate curve resolution (MCR) to determine the number of components, calculate component concentrations, and generate result vectors that are used to identify components through library searches.
- Apply the OMNIC Atlμs image analysis feature to digitize the concentration maps into binary maps of the constituents.
- Calculate the area occupied by each sample constituent from the binary maps.
- Convert the percent surface area of each component to percent weight.
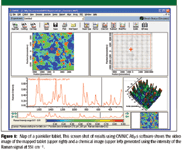
Figure 1
A commercially available, over-the-counter painkiller tablet with surface coating was sliced in half. The dispersive Raman microscope equipped with a 10x objective was used to map the cut surface of the tablet using the 780-nm excitation laser and the imaging software. Raman spectra over the fingerprint region (200–1800 cm-1 ) were collected from a 6 mm × 5.5 mm area at 50-μm intervals. A total of 13,542 spectra were collected. Spectral preprocessing consisted of a baseline correction, followed by normalizing the maximum peak intensity in each spectrum to unity.
Results
Figure 1 is a screen shot showing the results obtained using the mapping software. The figure shows a video image of the tablet surface and a chemical image based upon the Raman intensity at 551 cm-1 . It also shows the Raman spectrum acquired at the position in the video and chemical images that is marked with the red cross-hairs. The chemical map shows three distinct areas. A spectral library search on representative spectra from each area suggested that the three areas represented the distribution of caffeine, aspirin, and acetaminophen. However, because the step size in the map is 50 μm and the laser spot size with the 10X objective is 10–20 μm, spectra from many pixels in the map are likely to show spectral features corresponding to more than one component. This precludes using simple Raman intensity maps such as this for quantitative analysis.
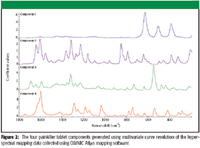
Figure 2
It is possible to extract the pure component spectra by using the MCR function incorporated into the mapping software. MCR is a statistical analysis procedure that deconvolutes the spectral data in the sample space into the product of a pure spectral matrix and a concentration matrix. The resulting spectral components are shown in Figure 2. Three of the resulting vectors generated by MCR were very similar to the spectra of the three components already identified by the library search. In addition, a fourth component was identified. The unknown component (Figure 2, component 1) showed three characteristic peaks in the low frequency region. A library search identified this component as titanium dioxide, which is used frequently for whitening and opacity in formulation coating materials. The remaining components were confirmed to be acetaminophen, caffeine, and aspirin. One advantage of using MCR is that the resulting components are sufficiently free from cross-contamination by other components that they usually can be identified by performing a library search.
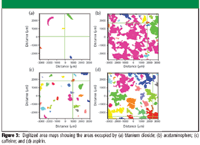
Figure 3
The distribution of the four components can be displayed as concentration maps. Using image analysis features in the software, the maps of the four components were digitized to generate binary images (Figure 3). Assuming the densities of the four components are the same, the relative concentration of each can be calculated from the relative areas in the binary maps. The results are shown in Table I and are comparable to the reported percent by weight for the three main components. The advantage of using MCR rather than trial-and-error inspection of spectra to identify the sample components is that MCR makes it much easier to identify minor contaminants. This is illustrated in Figure 4. The spectrum in Figure 4a is of pure aspirin, whereas that in Figure 4b is from aspirin contaminated with 5% TiO2. Without prior knowledge, it would be difficult to detect and identify the contaminant.
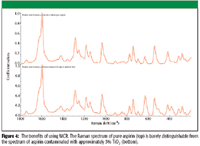
Figure 4
Summary
A method has now been established to achieve calibrationless semiquantitative analysis of a heterogeneous sample by using a dispersive Raman microscope to map the surface of a painkiller tablet. The MCR analysis and library searching capabilities of combined spectroscopy and mapping software were used to identify the tablet components. From the digitized concentration maps generated using image analysis, the relative areas and thus the relative concentrations of the tablet components were calculated. The calculated results were in good agreement with those provided by the tablet manufacturer. By revealing the presence and obtaining the spectrum of a minor impurity, the results clearly demonstrate the benefit of using multivariate curve resolution in this application.

Table I: Percent by weight composition of painkiller tablet calculated from the binary image maps and compared with the reported formulation weight percent
Koichi Nishikida is an applications specialist and Steve Lowry is a senior scientist with Thermo Electron Corporation, Madison, Wisconsin.
References
(1) K. Nishikida, J.A. Tarr, F. Izzia, and N.S. Nunn, "Standardless Semi-Quantitative Image Analysis of Heterogeneous Microscopic Materials," Poster, Pittsburgh Conference, Orlando, Florida, March 2006.

AI-Powered SERS Spectroscopy Breakthrough Boosts Safety of Medicinal Food Products
April 16th 2025A new deep learning-enhanced spectroscopic platform—SERSome—developed by researchers in China and Finland, identifies medicinal and edible homologs (MEHs) with 98% accuracy. This innovation could revolutionize safety and quality control in the growing MEH market.
New Raman Spectroscopy Method Enhances Real-Time Monitoring Across Fermentation Processes
April 15th 2025Researchers at Delft University of Technology have developed a novel method using single compound spectra to enhance the transferability and accuracy of Raman spectroscopy models for real-time fermentation monitoring.
Nanometer-Scale Studies Using Tip Enhanced Raman Spectroscopy
February 8th 2013Volker Deckert, the winner of the 2013 Charles Mann Award, is advancing the use of tip enhanced Raman spectroscopy (TERS) to push the lateral resolution of vibrational spectroscopy well below the Abbe limit, to achieve single-molecule sensitivity. Because the tip can be moved with sub-nanometer precision, structural information with unmatched spatial resolution can be achieved without the need of specific labels.