Fourier Transform-Infrared Spectroscopic Imaging: The Emerging Evolution from a Microscopy Tool to a Cancer Imaging Modality
The authors examine some of the novel biomedical applications of FT-IR imaging that are emerging today.

The integration of Fourier transform–infrared (FT-IR) spectroscopy with microscopy facilitates recording of spatially resolved spectral information, allowing the examination of both the structure and chemical composition of a heterogeneous material. While the first such attempt was over 50 years ago (1), present-day instrumentation largely evolved from the point microscopy detection of interferometric signals that developed in the mid-1980s (2). The successful coupling of interferometry for spectral recording and microscopy for spatial specificity in these systems spurred interest in a variety of fields, including the materials (3), forensic (4), and biomedical arenas (5,6). Point microscopy utilizes an aperture to restrict radiation incident on a sample and permits the recording of spatially localized data. The primary utilities of this form of microscopy lay in acquiring accurate spectra from small samples, in determining the chemical structure and composition of heterogeneous phases at specified points, and in building two-dimensional maps of the chemical composition of samples. Because the data were acquired at a single point, composition maps could only be acquired by rastering the sample. Hence, the approach was termed mapping or point mapping and involved as many interferometer scans as the number of pixels in the map.
The use of focal plane array (FPA) detectors for microscopy (7,8) allowed for the acquisition of large fields of view in a single interferogram acquisition sweep. The multichannel detection enabled by array detectors was similar to the concept of recording images with charge-coupled devices (CCDs) in optical microscopy; hence, the approach was termed imaging. The unique advantages of observing an entire field of view rapidly permitted applications that allowed monitoring of dynamic processes, spatially resolved spectroscopy of large samples or many samples, and enhanced quality due to retention of radiation throughput that was lost in point microscopy systems as a result of diffraction at the aperture. Just as for the previous generation of microspectroscopy instruments, applications rapidly followed in the materials (9) and biomedical fields (10–14). Research activity in this area can be divided into three major categories: instrumentation and sampling methodologies, applications, and data extraction algorithms. In this article, we review key advances and recent developments in the context of biomedical imaging. We do not provide a comprehensive overview but selectively highlight certain features of importance for cancer-related imaging. Last, we focus on one emerging application area, namely tissue histopathology, and provide illustrative examples from our laboratory indicating the integrative nature of protocol development.
Instrumentation, Sampling, and Data-Handling Techniques
Instrumentation
Because imaging is largely based upon new detectors with unique performance characteristics for spectroscopy, efforts in instrumentation have largely focused on the efficient integration of FPA detectors with interferometers. Because of the size, different electronics, and unique noise characteristics of FPAs, an optimization of data acquisition methodology was a primary activity in the initial period of availability of instrumentation. The first rational attempt at understanding performance and optimizing the data acquisition process revealed the unique noise characteristics that limited the first generation of array detectors (15). Briefly, this paper established that the general behavior of FT-IR spectrometers is generally held for imaging spectrometers but the detector may serve to limit the applicability of established practices in IR spectrometry. An explicit optimization of the data acquisition time revealed several strategies for speeding data collection for both the step-scan and rapid-scan modes (16). The first example of rapid-scan FT-IR imaging (17) was conducted using asynchronous sampling, followed by descriptions of synchronously triggered sampling and generalized methodologies (18) that could use any detector at any interferometer modulation frequency using post-acquisition techniques. Advances in detector technology have now allowed for rapid-scan imaging to become routine for large FPA detectors, while innovative new detectors have been developed (first by PerkinElmer, Shelton, Connecticut) that trade off a large multichannel detection advantage of arrays against the speed of smaller detector arrays to provide a very high-performance instrument (19).
At present, rapid-scan imaging has become the mode of choice for most manufacturers, and detector sizes have proliferated from the classic 64 × 64 format to range from 16 × 1 to 256 × 256 formats (Figure 1). Although the smaller detectors require rastering to image most samples and can provide data of higher quality more efficiently, larger detectors are generally employed for their large field of view and are useful for studying dynamics. It is interesting to note that the linear-array approach has an entirely different detector technology and considerations for electronics compared with the two-dimensional FPAs. Although it is beyond the scope of this article to discuss the differences, the use of "macro" electronics that are offset from the actual detector and ac mode of operation are the two major differences that affect data. Consequently, comparisons in performance are slightly more complicated. On the large format FPA front, the latest advance seems to be a detector developed jointly by NIH and FBI personnel in 2005. The detector can operate at 16 KHz for 128 × 128 FPA snaps (Bhargava, Schwartz, Levin, and Bartick, unpublished data). This is in the speed regime of single element detectors. Hence, the development can truly lead to the acquisition of an entire image in a single interferometer mirror sweep in the same time that it takes to acquire one spectrum with a benchtop IR spectrometer. To handle the large data output, we designed on-chip co-addition and various corrections. We believe that similar detector systems, operating in a fast regime and integrating processing with electronics, are likely to be the technology of tomorrow for FT-IR imaging.

Figure 1
The wide variety of instrumentation makes comparisons difficult, especially when manufacturers provide different specifications for instruments. We have proposed a comparison index for these systems based upon performance per unit time. Recognizing that spectral resolution, time for scanning, data processing (for example, apodization), and resultant image size are the primary determinants of performance, a measure can be formulated to describe performance. For a fixed data processing scheme (filtering, apodization, and so forth), the time taken to acquire 1 megapixel of data for 8 cm–1 resolution at a signal-to-noise ratio (S/N) of 1000:1 is found to be a good measure. We would like to emphasize that the performance is the performance of the entire imaging spectrometer and not due to the detector alone. Efficient coupling of the interferometer and optimization of the optical train will both affect performance as will the correct setup of the experiment. This index also does not consider the ease of use or "user-friendliness" of systems. These are other important considerations and must also be considered by organizations interested in FT-IR imaging technology.
The issue of time resolution for acquiring data is one such concern. The first approach is the kinetics approach, in which the interferometer is repeatedly scanned and imaging data sets are acquired sequentially as quickly as possible. Clearly, rapid scan is favored, and the availability of fast readout detectors is mandatory for fast events. The limit to this method is the readout speed of the array (frames in milliseconds), because interferometers generally can be scanned fast enough and the integration time required typically is in the tens of microseconds regime. An example is shown in Figure 2 to demonstrate applicability in monitoring polymerization kinetics.
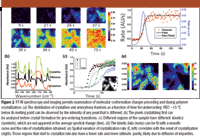
Figure 2
Though rapid scan imaging has displaced the step-scan mode in most new instrumentation, a very important application of the step-scan approach remains in time-resolved imaging (20–22). Briefly, the method is applicable to systems that can be repeatedly and reproducibly excited and relax back to their ground state. At each mirror retardation, the FPA is repeatedly triggered to acquire data. At the same time, the sample is excited once and the dynamics of excitation and decay of the excited state are monitored. Mirror stepping, data acquisition, and sample excitation are all synchronized precisely. Figure 3 demonstrates the synchronization. Time-resolved FT-IR imaging was first demonstrated using polymer–liquid crystal composites. Examples of the types of data that can be obtained are also shown in Figure 3. Last, the technology was extended to provide significantly higher time resolution than could be obtained by the electronics of the detector alone (23). Although FPA detectors are slow compared with single point detectors used in conventional FT-IR spectroscopy, the cause is the need to read out data from several thousand pixels and not from the need to record data from all pixels. Hence, by staggering the data recording time over multiple sample excitations, higher temporal resolution can be obtained. With current detectors, a time resolution of ~30 μs should be possible.
Figure 3 is not available. Please contact the author.
Sampling
Interferometer Issues
Among the sampling configurations, the first clearly was the optimization of the microscope for transmission and sampling. Unexpected issues were encountered in initial devices. For example, the detector for the monowavelength laser provides a fringe pattern to allow for tracking mirror retardation. The signal from this laser is measured by a small detector located at the center of the beamsplitter (to minimize errors), with an arm that extends out to the edge. When imaged onto the FPA, this laser detector leads to a pattern with low signal levels. Hence, the field of view is not uniform, leading, in turn, to lower S/N values for the affected region. Many manufacturers, hence, have redesigned their spectrometers for imaging use. Another manufacturer has avoided this issue by aligning its microscope to sample only the unaffected part of the beam. Because the issue was not important for nonimaging spectrometers, the interferometer was simply coupled to early microscopes and these issues were addressed slowly over time.
Sampling Modes: Transmission, Transmission-Reflection, Reflection, and Attenuated Total Reflectance
A vast majority of studies report the use of transmission sampling. Other major developments have been the incorporation of reflective slides (24–26), the integration of attenuated total reflectance (ATR) elements for both microscopy and large sample imaging, integration of ATR technology with various sample-forming accessories, grazing angle accessories, and multisample accessories. Reflective slides actually result in reflection-absorption that allows the beam to sample the signal twice, though with a different phase and lower signal due to half of the objective being used for transmitting light to the sample and the other half being used to acquire light from it. A detailed theoretical understanding of the confounding effects has not been published, though an example of the possible data correction algorithm has been reported by Diem and colleagues. ATR imaging is also highly prevalent and available as attachments to conventional imaging microscopes, using the sample chamber of the spectrometer and using it as a solid immersion lens (27). We discuss examples of ATR imaging next.
ATR
In the ATR mode, an IR transmitting crystal of precise geometry of high refractive index is employed as a solid immersion lens. Light is totally reflected at the sample–crystal interface and an evanescent field penetrates into the sample to provide the interaction to be observed using the traveling wave. Because the interaction is largely determined by the lens and not by the sample, precise and controlled depth of interaction is available. The sample, however, must be in good contact to allow efficient coupling with the evanescent wave. ATR imaging allows users to work with relatively thick sample sections that do not require much sample preparation expertise or time. The first use of ATR imaging was reported by Digilab (Canton, Massachusetts) in analyzing large samples that were not sectioned, as for transmission. ATR imaging microscopy was demonstrated soon after (28), followed by other novel accessories. There were other unpublished attempts that one of the authors is aware of: In 1999, for example, Snively and colleagues (personal communication, unpublished) demonstrated imaging data from an inverted ZnSe prism acting as a single bounce ATR. Soon after, we employed a Ge crystal but found the S/N of the imaging system of that time to be very poor. In addition to the ease of sample preparation, another major advantage of ATR imaging lies in improving the limited spatial resolution of transmission microscopy (29). The authors assessed that they were able to achieve a spatial resolution of 1 μm with a Ge internal reflection element.
Both micro and macro sampling have been utilized extensively (30). A spatial resolution of 3–4 μm using a Ge ATR element was claimed based on more stringent criteria than used previously (29). Ge, ZnSe, and diamond (30) crystals have been the materials of choice for most applications. In particular, Kazarian and co-workers (30–33) have extensively employed ATR–FT-IR imaging for various applications including drug release, polymer–drug formulations, and biological systems. The same group has provided other innovative sampling configurations for specific experiments, including a compaction cell that allows compaction of a tablet directly on a diamond crystal with a subsequent imaging (34). The changes in the distribution of a tablet consisting of hydroxypropyl methylcellulose and caffeine upon contact with water were studied. In this manner, conventional dissolution measurements were combined with a concurrent assessment of the compacted tablet structure (35). As opposed to the organic solvent–polymer dissolution experiments in transmission mode, this configuration allows for easy handling and imaging of water-induced dissolution. The setup also can provide high-throughput analysis of materials under controlled environments (36). A microdroplet sample deposition system was combined with a humidity control device to image about 100 samples deposited on the surface of an ATR crystal simultaneously. The approach was extended to 165 samples and reported to study parallel dissolution of formulations (37).
Multisample Accessories and Sampling
Although the structure of single samples has been the primary focus of FT-IR imaging, a number of applications utilize the technique to image multiple samples simultaneously. The first examples were from the field of catalyst research (38). Typically, 2–12 samples could be imaged and analyzed under the same conditions. High-throughput validation or method development was the primary goal in these studies. Tissue microarrays (TMAs) provide the same function in biomedical imaging. TMAs consist of tens to hundreds of samples arranged on a grid format. This allows for easy visualization of the structure and classification accuracy across many patients and the statistical measures needed for rigorous validation. The primary utility of the multisample image in this case is to provide wide-ranging sampling and convenient archiving or data storage, not necessarily to provide a higher throughput (14,39). With the appropriate geometry, many samples can be imaged to understand their dynamics in a concerted fashion. To accommodate the samples, the field of view is often expanded. This results in a lower spatial resolution. For imaging multiple samples, though, the spatial resolution can be conserved but temporal resolution is restricted.
Biomedical Applications
Bone
Bone has been the tissue studied most by FT-IR imaging. Bone composition changes with development, environment, genetics, health, and disease; is amenable to imaging at the resolution length scale of imaging; and has a limited chemical composition that is characterized using IR spectroscopy (40). For almost 30 years until the late 1980s (41), bone structure was studied using single-element detectors in FT-IR spectrometers. Typically, ground bone was analyzed using the conventional KBr pellet method. This pellet method obviously destroyed local structures, precluding an understanding of subtle variations as a result of disease. Nevertheless, it was sensitive to chemical composition and did provide useful information. With microscopy and now with FT-IR imaging, sample integrity is maintained and ability to acquire spectral information at anatomically discrete sites is possible. From the resulting spectra, several important pieces of information can be obtained. These include relative mixture composition of hydroxyapatite and collagen by calculating the ratio of the integrated ν1, ν3 phosphate and amide 1 (mineral:matrix ratio); carbonate substitution by calculating the ratio of carbonate/phosphate ratio from the ratio of integrated ν2 carbonate peak (850–900 cm–1 ) and ν1, ν3 phosphate contour (900–1200 cm–1 ); and crystallinity of the mineral phase from the ratio of 1030/1020 peak intensity (42). These assays illustrate several quantities important to bone research and disease diagnoses that can be performed readily. Though a complete discussion is available in the references (40,42–44), we pick three illustrative examples that demonstrate the applicability in disease and in research.
IR spectral analysis of healthy and diseased bone has been reviewed by Boskey and Mendelsohn (42), with particular emphasis on changes in bones composition, physiochemical status of mineral and matrix of bones during osteoporosis, and the effect of therapeutics on these parameters. Osteoporosis, or porous bone, is a bone disease characterized by low bone mass and structural deterioration of bone tissue. This leads to bone fragility and an increased susceptibility to fractures, especially at the hip, spine, and wrist. FT-IR images of the mineral content and crystallinity in trabecular bone of normal and osteoporotic samples clearly depicts that the trabeculae in diseased tissue are thinner. Moreover, the mineral/matrix ratio in osteoporotic bone is significantly reduced, whereas crystallinity is increased. These advances demonstrate the potential and applicability of the technique to characterize diseased tissue. Bone mineral changes between a healthy mouse model and Fabry diseased (lipid storage disease) mouse model were also analyzed in which globotriaosylceramide (Gb3) accumulates in tissues (43). No significant differences in the bone mineral properties were observed between Fabry and healthy mice, which might reflect the similar lack of major bone phenotype in human patients with Fabry's disease and could also be related to the developmental age of these animals. The study provides an example of the applicability to laboratory research.
Calcified tissue in biopsies from adults with osteomalacia has been studied (44). Osteomalacia results in a deficiency of the primary mineralization of the matrix, leading to an accumulation of osteoid tissue and reduction in bone's mechanical strength. A decrease in trabecular bone content with absence of changes in matrix or mineral is noticed when iliac crest biopsies of individuals with vitamin D–deficient osteomalacia are compared to normal controls. These findings support the assumption that, in osteomalacia, the quality of the organic matrix and of mineral in the center of the bone does not vary, whereas less-than-optimal mineralization occurs at the bone surface.
Brain
Monkey brain tissues were among the first tissues examined by using FT-IR imaging (12). Lately, the applications have experienced a renaissance with applications to the human brain. Grossly, the brain can be divided into two types of matter, namely gray matter and white matter. These names derive simply from their appearance to the naked eye. Gray matter consists of cell bodies of nerve cells, while white matter consists of the long filaments that extend from the cell bodies — the "telephone wires" of the neuronal network, transmitting the electrical signals that carry the messages between neurons. A visualization of the two compartments formed the first demonstrative application of FT-IR microspectroscopic imaging.
FT-IR imaging and multivariate statistical analyses (unsupervised hierarchical cluster analysis) were applied along with histology and immunohistochemistry in an animal model having glioblastoma multiform (GBM) (45). GBM is a highly malignant human brain tumor that is considered to be the one of the most difficult to treat effectively (46). Authors were able to identify the tumor growth as chemically distinct from the surrounding brain tissue. The distribution of the absorbance of amide I in images highlighted high concentrations of proteins in the corpus callosum and regions of basal ganglia for healthy brain. Low absorbance generally was observed in the cortex, while a higher absorbance was observed at the outer layer of the cortex. For a GBM-bearing animal, the highest absorbance was found at the tumor site. In contrast to healthy brain, a lower absorbance of the amide I band was observed at the corpus callosum when compared to that in the cortex and the caudoputamen. The study demonstrates a powerful application of simple analyses that can indicate disease. It also highlights the multitude of spatial and spectral clues that can be used to diagnose or understand the disease.
In addition to primary disease sites, diagnoses of metastatic spread from various cancers also were reported (47). A multivariate classification algorithm was used to distinguish normal tissue from brain metastases successfully and to classify the primary tumor of brain metastases from renal cell carcinoma, lung cancer, colorectal cancer, and breast cancer. In the cluster-averaged IR spectra from a brain metastasis of renal cell carcinoma, the main spectral differences were observed for the three tissue regions in the region from 950 to 1200 cm–1 and from 1500 to 1700 cm–1. Band intensities of 1026, 1080, and 1153 cm–1 are at maximum in the spectrum of black cluster and at minimum in the spectrum of light gray cluster. The comparisons of the IR spectra of normal brain tissue and brain metastases of lung, breast cancer, and colorectal cancer were made; these spectra do not contain spectral features at 1026, 1080, and 1153 cm–1 that are indicative of the presence of glycogen. It was concluded that these aforementioned spectral features would be considered as biomarkers for brain metastases of the primary tumor renal cell carcinoma. In addition to these three bands, spectral differences were observed for the bands at 1542 and 1655 cm–1, owing to the presence of amide I and amide II vibrations. It is clear from the results that the maximum protein concentrations correlate with minimum glycogen concentrations in the IR image. However, the protein and glycogen properties evident in the IR image are not visible in the unstained cryosection. It is noteworthy that simple univariate analyses provide the end clues to the disease. Even on application of multivariate techniques, the most prominent and easy to understand biomarkers of disease are those defined by conventional spectroscopic knowledge as being important for identification, namely, features and their absorption.
In the cluster-averaged IR spectra of white matter from the three normal brain tissue samples, intense bands at 1060, 1233, 1466, 1735, 2850, and 2920 cm–1 due to the high lipid concentration in white matter were noticed. Intensity changes were due to intersample and patient-to-patient variances of the same tissue type. In addition, cluster-averaged IR spectra of a brain metastasis of (renal cell carcinoma, breast cancer, lung cancer, and colorectal cancer) and gray matter of normal brain tissue were compared after baseline subtraction and then normalization with respect to the amide I band. Significant differences in the band positions, intensities, and area were observed between these samples, which were then used as potential candidates to differentiate normal and tumor tissue and for the identification of the primary tumor. Here, the authors used only eight spectroscopic features for the linear discriminant analysis model. They were able to classify correctly for three out of three normal brain tissue and 16 out of 17 brain metastases samples. Hence, though univariate analyses and features provide useful recognition, their integration into a multivariate algorithm provides for automated recognition of clinical importance. It can also be argued, however, that it is questionable whether the small numbers of samples employed represent a true performance condition for the algorithm or are simply reflective of bias arising from the clinical setting or sample sources (78). The advent of faster imaging approaches and advanced sampling techniques like TMAs can allow for larger numbers of samples to be analyzed and such doubts about the validity of studies to be put to rest.
Similarly, tissues from rat glioma models have been characterized and used to discriminate healthy from tumor sections using principal component analysis and K-means (48). Pseudo color maps reported were constructed on 8-means clusters, where each cluster consists of similar spectra. The lipid/protein ratio (1466/1452 cm–1 ) was found to be decreased and the band at 1740 cm–1 became weak and almost vanished as compared with the corresponding bands in the healthy tissue. In addition to the above-mentioned differences, significant differences between healthy and tumor-affected tissue were observed in the fingerprint region. In the healthy tissue, a weak band at 1172 cm–1 , representing the stretching mode of C-O groups, was observed. Reduced intensity as well as shifting of peak to 1190 cm–1 was noted for tumor and surrounding tumor spectra. Tumor tissue was observed to contain a decreased intensity of the asymmetric phosphate stretching and C-C stretching and an increased intensity of the symmetric phosphate stretching when compared to the healthy tissue. Variations in lipid features (methylene and methyl stretching) also were observed. The major point here is that the entire spectrum contains numerous points of difference between healthy and diseased tissue. The results agreed with those obtained from pathology (49). The structural difference around the tumor was noted, which could be ascribed to the peritumoral aedoma observed during glioma development. An increase in the permeability of the blood–brain barrier and aggravation in the mass effect of tumors are the rationale for aedoma, which is associated with brain tumor. Fundamental understanding can be enhanced by a detailed analysis of the spectral differences, but prediction algorithms need only a few measures of the spectral data to be effective.
Breast
Two major applications in breast tissue deal with complications arising from artificial alterations of the tissue and the evolution of cancer. Although breast augmentation with implants is highly prevalent, its complications have been discussed more recently. On the other hand, the conventional method for diagnosing and evaluating the prediction of breast disease is a histopathological examination of biopsy samples, a practice that has some shortcomings. For breast implants, a major question is the containment of filling material, because its leakage can lead to potential diseases. The silicone gel in implants is very different chemically from surrounding tissue, and its presence in tissue sections indicates a definite leak from the implant either due to material failure or as a consequence of aging. A spectroscopic image (50) generated from the asymmetric stretching modes of the methyl groups attached to silicon in the gel allowed for the examination of silicone in the tissue. Because of the unique chemical contrast employed in FT-IR imaging, such presence can be discerned within the tissue, even when optical microscopy contrast was poor. An example of the presence of Dacron (a commercial name for poly[ethylene terepthalate]) fixative patch threads in breast tissues was shown (50). It was noted that the technique is capable of rapid analysis within minutes of sectioning the tissue.
A few reports also have applied FT-IR imaging for diagnosing breast diseases. Breast tumor tissues were characterized by both FT-IR imaging and point mapping techniques, and advantages over the other were evaluated (51). Similar comparisons previously had been reported for polymeric materials, analyzing both static and dynamic samples (52). Comparing images from the two methods, imaging data provided a clearer structure in the tumor area than the data obtained from point mapping. Because breast tumor cells are ~10 μm in diameter, point mapping data (with an aperture of 30 μm) would always contain the spectrum of tumor cells as well as the contributions of other components surrounding the cells. The study clearly indicated that the conventional point mapping approach could fail to detect a small number of malignant cells as a result of its poor resolution capabilities. Nevertheless, the contamination problem (that is, the spectral contributions of other components surrounding the cell) is found to be less severe in the case of ductal carcinoma in situ (DCIS). The study illustrates the need for matching the appropriate level of spatial resolution to the task. Although the 30-μm resolution might be appropriate for some applications, it was clearly insufficient for detecting smaller numbers of cells.
Artificial network and K-means cluster analysis also have been employed for the classification of FT-IR imaging data from normal and malignant immortalized human breast cell lines (53). Normal cells, carcinoma cells, and mixed normal and carcinoma cells were used. Differences in the spectral backgrounds between the training and test data were observed, which confounds the reproducibility of recorded spectra and, thus, causes the classifier to fail. Using rejection thresholds in the application of the artificial neural network (ANN) classifier was reported to be helpful in identifying doubtful classifications. Another study (54) reported imaging fibroadenoma, a benign breast tumor. Data were evaluated using unsupervised cluster analysis by utilizing two spectral regions, namely 1000–1500 and 2800–3000 cm–1 . The distributions of four main tissue components — epithelium, retro nuclear basal epithelial regions, mantle zone, and distant connective tissue — were visualized. The spectral features from each component were discussed in detail. Furthermore, comparing epithelia from fibroadenoma and DCIS, the authors determined that subtle distinctions between the IR characteristics of these two are reproducible. The initial study used tissue from a single patient.
The work was recently extended (55) to diagnose benign and malignant lesions from 22 patients. The study utilized only spectra from well-defined tumor areas, owing to the heterogeneity of tissues. Based upon the cluster analysis and on comparison with the H&E (hematoxylin and eosin) images, four classes of distinct breast tissue spectra were identified — fibroadenoma, DCIS, connective tissue, and adipose tissue. Further, ANNs were developed as an automated classifier to differentiate the four classes. All spectra of connective tissue and adipose tissue were classified correctly, where the spectral features are clearly different from each other and from tumors as well. Differentiating fibroadenoma from DCIS was more difficult. A toplevel–sublevel strategy was further applied and was able to differentiate 93% between fibroadenoma and DCIS spectra by employing principal component analysis. From the mean spectra, it was found that the DCIS has more lipid content than the fibroadenoma. Invasive ductal carcinoma (IDC) could not be well characterized due to contamination from surrounding cells, illustrating the limited spatial resolution.
Cervical Cancer
The cervix is the lower part of the uterus (womb) in which two major types of cancers occur: squamous cell carcinoma and adenocarcinoma. About 80–90% of cervical cancers are squamous cell carcinomas, and the remaining 10–20% are adenocarcinomas. Less commonly, cervical cancers have features of both squamous cell carcinomas and adenocarcinomas. These are called adenosquamous carcinomas or mixed carcinomas. Typically, the Papanicolaou (Pap) test checks for changes in the exfoliated cells of the cervix to find the presence of any infection, abnormal (unhealthy) cervical cells, or cervical cancer. FT-IR spectroscopy, microspectroscopy, and FT-IR imaging have been widely utilized to study cervical cancer and to perform the same function using computer analyses of spectra (26,56–60). While the first reports in diagnosing cervical cancer are now generally not regarded as leading to solutions (56), two groups have provided definitive proof of the potential of IR spectroscopy by careful microscopy studies (26,45,57,59,60). While FT-IR images of the amide I and υasy PO2– bands with H&E stained image were compared and only a rough correlation with the pathological features or cell types were obtained, cluster maps of two, five, and eight clusters resulting from UHC analysis for the whole spectrum demonstrated good segmentation. In five clusters, most cell types are apparent, including superficial, intermediate, parabasal, and connective tissue upon correlation with the stained image. As in univariate images, the connective tissue region is split into two clusters. Furthermore, by comparing the unsupervised hierarchical clustering (UHC) analysis of the whole spectrum and only the amide I region, authors demonstrated that minimizing the spectral region for analysis and using fewer clusters does not lead to the loss of useful information. Both univariate FT-IR and multivariate images of the sample with several endocervical ducts within the connective tissue were shown. These endocervical ducts lined with columnar endocervical cells were apparent in all those images, in particular even with two clusters.
Cultures derived from cervical cancer cells (HeLa) are one of the most popular model systems and have been studied using FT-IR imaging (61). The cells were grown directly as sparse monolayers onto low-e slides. FT-IR image of amide I band region was shown, where large differences in spectral intensities associated with the cells were observed even though these cells are from a homogeneous and exponential cell culture. Cluster analyses of normalized spectra show distinct differences that were not appreciated in the univariate image. Similarly (62), IR imaging with fuzzy C-means (FCM) clustering and hierarchical cluster analysis were utilized to study the thin sections of cervix uteri encompassing normal, precancerous, and squamous cell carcinoma. These studies demonstrate that IR imaging, in combination with multivariate techniques, is capable of segmenting cervical tissues in a manner that is comparable to H&E stained image differentiation and is significantly more sensitive in terms of the chemical composition of the cells – whether it be due to metabolic or disease reasons.
Prostate
Prostate cancer is the most prevalent internal cancer in the US (63). Hence, its pathologic diagnosis and correct interpretation of disease state are crucial (64). FT-IR imaging has been proposed as a solution that can potentially help pathologists by providing an objective and reproducible assessment of disease in a manner that is easily understood by clinicians. It is also a good model system for the development of FT-IR imaging protocols. We first review progress in the field and then describe efforts in our and collaborator's laboratories toward formulating a practical algorithm for prostate cancer pathology. While a number of studies examined human prostate tissue with IR spectroscopy (65–68), microscopy approaches have recently been extensively utilized to study fundamental properties of prostate tissue and to determine structural units in normal and disease states (69–75). An understanding of the tissue is now emerging as a result of these studies. Although the fundamental properties of the tissue are being examined, we have focused on developing statistically validated diagnostic methods.
We have used high-throughput imaging with the express purpose of correlating spectra to clinical practice (39,64,76). It is instructive first to examine the approaches of some previous studies and then describe our approach in some detail. A variety of techniques have been reported for analyzing prostate tissue, including unsupervised multivariate data analysis techniques such as agglomerative hierarchical clustering (AH), FCM, or k-means (KM) clustering to construct infrared spectral maps of tissue structures (77). The results from these multivariate techniques confirmed the standard histopathological techniques and were helpful for identifying and discriminating the tissue structures. Agglomerative hierarchical clustering was found to be the best method among the cluster imaging methods in terms of segmenting the tissue. Although these techniques comprise one end of the approach in using large spectral regions and completely objective methods, the other extreme also has proven to be useful. In the second paradigm, careful examination of the spectral data yields some measures that prove useful. For example, the ratio of peak areas at 1030 and 1080 cm–1, corresponding to the glycogen and phosphate vibrations, respectively, was utilized as a diagnostic marker for the differentiation of benign from malignant cells (69). Authors summarized that the use of this ratio in association with FT-IR spectral imaging provides a basis for estimating areas of malignant tissue within defined regions of a specimen. While it can be argued that the former is not based upon clinical knowledge and is more suited for discovery, it also involves the choice of selecting specific numbers of clusters and their subsequent interpretation. The latter is based upon a single parameter whose utility for universal diagnoses remains to be tested. Nevertheless, these studies indicate that both approaches provide information about the tissue that is useful.
Our approach has used elements from both pattern recognition and spectroscopic analyses of univariate measures (39,76). In all cases, one starts with the acquired imaging data (Figure 4). Because the data set is large (typically 10–1000 GB), it is advisable to reduce the dimensionality of data using some numerical procedure. Compression algorithms, principal components analyses, or simply storing only the information needed for classification (if the algorithm is known) are useful. We sought expressly to relate the recorded IR imaging data to a clinical knowledge base. Hence, we started with a model that is derived from clinical practice. Clearly, the approach limits the discovery of new knowledge, but it assures the clinician that all quantities of importance for diagnoses will be considered. The acquired data is labeled with known cell identity or disease states. These pixels are best identified by a combination of very careful manual labeling and testing for absorbance fidelity (78). Spectra from the labeled regions are employed via average values, medians, and standard deviation analyses to determine a set of spectral features that are descriptive of the major features of all spectra. We first note that the characteristic IR absorbance spectra of 10 histological classes comprising prostate tissue look similar. Though small differences in spectral features were observed at many frequencies, summary statistics are limited in their examination of spectra for classification. Further, the small differences indicate that noise and biological variability may render univariate measures less reliable. The large number of classes usually implies that univariate analyses cannot distinguish all histological classes present in the tissues and hence the need for multivariate analyses is apparent. Here, the similarity of the spectral features for all classes works in our favor. Very similar baseline points are obtained from an analysis of all spectra and only subtle feature differences are noted to distinguish the various class spectra. Hence, unknown spectra can be processed in the same specified manner, without introducing any bias. Each of these features is termed a metric to denote that it is a useful measure of the spectrum. Individual metrics can allow segmentation of various tissue types if they are sufficiently different in a sampled population.
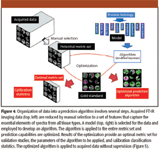
Figure 4
We then employ the equivalent of a t-test in that the overlap between the absorbance distributions of metrics is determined and equated to the error in prediction. The metrics are arranged in the order of increasing overlap. Hence, we have an ordered set that differentiates at least two classes. To obtain overall accuracy, we employ a modified Bayesian algorithm to provide the probability of each class for every pixel. This fuzzy result is employed to determine the area under the curve (AUC) of a receiver operating characteristic (ROC) curve. The ROC curve is built from accepting the probability of each class at an increasing threshold that varies between 0 and 1. For optimized threshold values, the fuzzy classification is turned into a classified image, where each pixel is assigned a distinct class. We note that the method incorporates analysis of all spectral features, a selection of the best features based upon statistical analysis of data and an optimal prediction of the class of each pixel based upon an objective selection rule from the fuzzy classification. The method is very powerful in that it employs spectral features that are ordinarily employed by spectroscopists as metrics, which permits a spectroscopic analysis of the basis of decision-making. Further, the method explicitly obtains the fuzzy rule data for final classification. The value of the rule data for each class is actually the probability of belonging to the class without consideration for the prior prevalence of the class. Hence, the method can allow direct comparisons between performances for different classes. The dependence of the process on various experimental parameters also has been reported (64).
The complication inherent in translating the results from a small data set of patients to clinical applications is well recognized in the spectroscopy community. The variability in data, arising from variations within and between patients, sample preparation, and handling, is likely to provide noisy estimates of performance. Hence, statistical stability may be obtained by examining a large number of samples. Similarly, large numbers of patients may be employed to provide calibration models, likely improving the robustness of the developed algorithm. We have described a high-throughput sampling method from tissues (14,39,76). Briefly, the approach uses a combinatorial sampling of tissue type and pathology to first acquire small sections of tissues from large archival cases. These small sections are arranged in a grid pattern and placed on the same substrate. The sample is termed a tissue microarray to reflect the similarity with cDNA microarrays. For spectroscopic imaging and the development of automated algorithms, the approach represents a large number of cases that can be used both for accurate prediction algorithm building and for extensive validations. The same approach is likely to prove useful for extensions to determining pathology. Figure 5 demonstrates the typical workflow of a validation algorithm and methods used for statistical comparison. We strongly suggest a variety of methods for measuring performance because each method has its own advantages and disadvantages. For example, summary measures from ROC curves provide information about accuracy but do not indicate which class the inaccuracies arise from. Similarly, confusion matrices provide cross-class information but do not provide global performance measures in the mold of ROC curves.
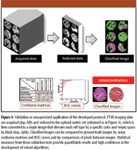
Figure 5
Outlook
FT-IR imaging has experienced rapid growth in the past 10 years and is increasingly being applied to biomedical tissue, especially for the analyses of cancer. The major trends emerging in instrumentation include faster detectors and novel modes of data collection (for example, time-resolved imaging), of sampling (for example, ATR), and application areas. For biomedical samples, the information content is quite rich and is often available through simple univariate analysis. For more complex applications such as cancer diagnoses, the data acquisition, sampling, and data analyses must be integrated in a coherent manner to provide a practical solution. We anticipate that the technology and its application to biomedical problems will continue to grow with the cooperation of instrument manufacturers, applications scientists, numerical methods developers, and communities that can utilize the information effectively, such as pathologists or surgeons.
Gokulakrishnan Srinivasan and Rohit Bhargava are with the Department of Bioengineering and Beckman Institute for Advanced Science and Technology, University of Illinois at Urbana-Champaign, Urbana, Illinois.
References
(1) K.P. Norris, J. Sci. Inst. 31, 284–287 (1954).
(2) J.M. Kwiatkoski and J.A. Reffner, Nature 328(6133), 837–838 (1987).
(3) J.L. Koenig, Microspectroscopic Imaging of Polymers (American Chemical Society, Washington, DC, 1998).
(4) D.K. Williams, R.L. Schwartz, and E.G. Bartick, Appl. Spectrosc. 58(3), 313–316 (2004).
(5) W. Petrich, Appl. Spectrosc. Reviews 36(2–3), 181–237 (2001).
(6) D. Naumann, Appl. Spectrosc. Reviews 36(2–3), 239–298 (2001).
(7) E.N. Lewis and I.W. Levin, Microscopy and Microanalysis 1(01), 35–46 (1995).
(8) E.N. Lewis, P.J. Treado, R.C. Reeder, G.M. Story, A.E. Dowrey, C. Marcott, and I.W. Levin, Anal. Chem. 67(19), 3377–3381 (1995).
(9) R. Bhargava, S.Q. Wang, and J.L. Koenig, Adv. Polym. Sci. 163, 137–191 (2003).
(10) R. Mendelsohn, C.R. Flach, and D.J. Moore, Biochim. Biophys. Acta – Biomembranes 1758(7), 923–933 (2006).
(11) P. Garidel and M. Boese, Microsc. Res. Tech. 70(4), 336–349 (2007).
(12) E.N. Lewis, A.M. Gorbach, C. Marcott, and I.W. Levin, Appl. Spectrosc. 50(2), 263–269 (1996).
(13) E.N. Lewis, L.H. Kidder, I.W. Levin, V.F. Kalasinsky, J.P. Hanig, and D.S. Lester, Ann. N.Y. Acad. Sci. 820, 234–246; discussion 246–247 (1997).
(14) I.W. Levin and R. Bhargava, Annu. Rev. Phys. Chem. 56, 429–474 (2005).
(15) C.M. Snively and J.L. Koenig, Appl. Spectrosc. 53(2), 170–177 (1999).
(16) R. Bhargava and I.W. Levin, Anal. Chem. 73(21), 5157–5167 (2001).
(17) C.M. Snively, S. Katzenberger, G. Oskarsdottir, and J. Lauterbach, Opt. Lett. 24, 1841–1843 (1999).
(18) S.W. Huffman, R. Bhargava, and I.W. Levin, Appl. Spectrosc. 56(8), 965–969 (2002).
(19) Spectrochemical Analysis Using Infrared Multichannel Detectors, R. Bhargava and I.W. Levin, Eds. (Blackwell Publishing, Sheffield, UK, 2005).
(20) R. Bhargava and I.W. Levin, Appl. Spectrosc. 57(4), 357–366 (2003).
(21) R. Bhargava and I.W. Levin, Macromolecules 36(1), 92–96 (2003).
(22) R. Bhargava and I.W. Levin, Appl. Spectrosc. 58(8), 995–1000 (2004).
(23) R. Bhargava and I.W. Levin, J. Phys. Chem. A 108(18), 3896–3901 (2004).
(24) T.J. O'Leary, W.F. Engler, and K.M. Ventre, Appl. Spectrosc. 43(6), 1095–1097 (1989).
(25) C. Marcott, G.M. Story, and R.K. Dukor, Microscopy and Microanalysis 10(S02), 182–183 (2004).
(26) M. Romeo, B. Mohlenhoff, M. Jennings, and M. Diem, Biochim. Biophys. Acta – Biomembranes 1758(7), 915–922 (2006).
(27) B.M. Patterson and G.J. Havrilla, Appl. Spectrosc. 60(11), 1256–1266 (2006).
(28) A.J. Sommer, L.G. Tisinger, C. Marcott, and G.M. Story, Appl. Spectrosc. 55(3), 252–256 (2001).
(29) D.B. Otts, P. Zhang, and M.W. Urban, Langmuir 18(17), 6473–6477 (2002).
(30) K.L.A. Chan and S.G. Kazarian, Appl. Spectrosc. 57(4), 381–389 (2003).
(31) K.L. Chan, S.V. Hammond, and S.G. Kazarian, Anal. Chem. 75(9), 2140–2146 (2003).
(32) C.S. Colley, S.G. Kazarian, P.D. Weinberg, and M.J. Lever, Biopolymers 74(4), 328–335 (2004).
(33) S.G. Kazarian and K.L.A. Chan, Biochim. Biophys. Acta — Biomembranes 1758(7), 858–867 (2006).
(34) J. van der Weerd, K.L.A. Chan, and S.G. Kazarian, Vib. Spectrosc. 35(1–2), 9–13 (2004).
(35) J. van der Weerd and S.G. Kazarian, J. Controlled Release 98(2), 295–305 (2004).
(36) K.L. Chan and S.G. Kazarian, J. Comb. Chem. 7(2), 185–189 (2005).
(37) K.L. Chan and S.G. Kazarian, Lab. Chip. 6(7), 864–870 (2006).
(38) C.M. Snively, G. Oskarsdottir, and J. Lauterbach, Catal. Today 67(4), 357–368 (2001).
(39) D.C. Fernandez, R. Bhargava, S.M. Hewitt, and I.W. Levin, Nat. Biotechnol. 23(4), 469–474 (2005).
(40) A.L. Boskey and R. Mendelsohn, Vib. Spectrosc. 38(1–2), 107–114 (2005).
(41) A.S. Posner and G. Duyckaerts, Experientia 10(10), 424–425 (1954).
(42) A. Boskey and R. Mendelsohn, J. Biomed. Opt. 10(3), 031102 (2005).
(43) A.L. Boskey, M. Goldberg, A. Kulkarni, and S. Gomez, Biochim. Biophys. Acta — Biomembranes 1758(7), 942–947 (2006).
(44) D. Faibish, A. Gomes, G. Boivin, I. Binderman, and A. Boskey, Bone 36(1), 6–12 (2005).
(45) K.R. Bambery, E. Schultke, B.R. Wood, S.T.R. MacDonald, K. Ataelmannan, R.W. Griebel, B.H.J. Juurlink, and D. McNaughton, Biochim. Biophys. Acta — Biomembranes 1758(7), 900–907 (2006).
(46) American Brain Tumor Association.
(47) C. Krafft, L. Shapoval, S.B. Sobottka, G. Schackert, and R. Salzer, Technol. Cancer Res. Treat. 5(3), 291–298 (2006).
(48) N. Amharref, A. Beljebbar, S. Dukic, L. Venteo, L. Schneider, M. Pluot, R. Vistelle, and M. Manfait, Biochim. Biophys. Acta — Biomembranes 1758(7), 892–899 (2006).
(49) J. Kneipp, P. Lasch, E. Baldauf, M. Beekes, and D. Naumann, Biochim. Biophys. Acta — Molecular Basis of Disease 1501(2–3), 189–199 (2000).
(50) L.H. Kidder, V.F. Kalasinsky, J.L. Luke, I.W. Levin, and E.N. Lewis, Nat. Med. 3(2), 235–237 (1997).
(51) H. Fabian, P. Lasch, M. Boese, and W. Haensch, Biopolymers — Biospectroscopy Section 67(4–5), 354–357 (2002).
(52) R. Bhargava, B.G. Wall, and J.L. Koenig, Appl. Spectrosc. 54, 470–479 (2000).
(53) L. Zhang, G.W. Small, A.S. Haka, L.H. Kidder, E.N. Lewis, Appl. Spectrosc. 57(1), 14–22 (2003).
(54) H. Fabian, P. Lasch, M. Boese, and W. Haensch, J. Mol. Struct. 661–662(1–3), 411–417 (2003).
(55) H. Fabian, N.A.N. Thi, M. Eiden, P. Lasch, J. Schmitt, and D. Naumann, Biochim. Biophys. Acta — Biomembranes 1758(7), 874–882 (2006).
(56) P.T. Wong, R.K. Wong, T.A. Caputo, T.A. Godwin, and B. Rigas, Proc. Natl. Acad. Sci. U.S.A. 88(24), 10988–10992 (1991).
(57) S. Boydston-White, M. Romeo, T. Chernenko, A. Regina, M. Miljkovic, and M. Diem, Biochim. Biophys. Acta —Biomembranes 1758(7), 908–914 (2006).
(58) M.J. Walsh, M.J. German, M. Singh, H.M. Pollock, A. Hammiche, M. Kyrgiou, H.F. Stringfellow, E. Paraskevaidis, P.L. Martin-Hirsch, and F.L. Martin, Cancer Lett. 246(1–2), 1–11 (2007).
(59) K.R. Bambery, B.R. Wood, M.A. Quinn, and D. McNaughton, Aust. J. Chem. 57(12), 1139–1143 (2004).
(60) B.R. Wood, L. Chiriboga, H. Yee, M.A. Quinn, D. McNaughton, and M. Diem, Gynecol. Oncol. 93(1), 59–68 (2004).
(61) M. Diem, M.J. Romeo, S. Boydston-White, and C. Matthaus, "IR Spectroscopic Imaging: From Cells to Tissue" in Spectrochemical Analysis Using Infrared Multichannel Detectors, R. Bhargava and I.W. Levin, Eds. (Blackwell Publishing, Sheffield, UK, 2005).
(62) W. Steller, J. Einenkel, L.C. Horn, U.D. Braumann, H. Binder, R. Salzer, and C. Krafft, Anal. Bioanal. Chem. 384(1), 145–154 (2006).
(63) http://seer.cancer.gov/csr/1975_2004/results_single/sect_01_table.01.pdf. 2007.
(64) R. Bhargava, Anal. Bioanal. Chem., submitted for publication.
(65) C. Paluszkiewicz and W.M. Kwiatek, J. Mol. Struct. 565, 329–334 (2001).
(66) R. Bhargava, D.C. Fernandez, M.D. Schaeberle, and I.W. Levin, Appl. Spectrosc. 55(12), 1580–1589 (2001).
(67) H.S. Hsu, S.Y. Lin, M.J. Li, and R.C. Liang, Ultrastructural Pathology 26(3), 137–141 (2002).
(68) M.J. Li, H.S. Hsu, R.C. Liang, and S.Y. Lin, Ultrastructural Pathology 26(6), 365–370 (2002).
(69) E. Gazi, J. Dwyer, P. Gardner, A. Ghanbari-Siahkali, A.P. Wade, J. Miyan, N.P. Lockyer, J.C. Vickerman, N.W. Clarke, J.H. Shanks, L.J. Scott, C.A. Hart, and M. Brown, J. Pathol. 201(1), 99–108 (2003).
(70) E. Gazi, J. Dwyer, N. Lockyer, P. Gardner, J.C. Vickerman, J. Miyan, C.A. Hart, M. Brown, J.H. Shanks, and N. Clarke, Faraday Discuss. 126, 41–59; discussion 77–92 (2004).
(71) E. Gazi, J. Dwyer, N.P. Lockyer, J. Miyan, P. Gardner, C. Hart, M. Brown, and N.W. Clarke, Biopolymers 77(1), 18–30 (2005).
(72) E. Gazi, J. Dwyer, N. Lockyer, P. Gardner, J. Miyan, C. Hart, M. Brown, and N. Clarke, Vib. Spectrosc. 38(1–2), 193–201 (2005).
(73) M.J. German, A. Hammiche, N. Ragavan, M.J. Tobin, L.J. Cooper, S.S. Matanhelia, A.C. Hindley, C.M. Nicholson, N.J. Fullwood, H.M. Pollock, and F.L. Martin, Biophys. J. 90(10), 3783–3795 (2006).
(74) E. Gazi, M. Baker, J. Dwyer, N.P. Lockyer, P. Gardner, J.H. Shanks, R.S. Reeve, C.A. Hart, N.W. Clarke, and M.D. Brown, Eur. Urology 50(4), 750–761 (2006).
(75) W.F. Wolkers, S.K. Balasubramanian, E.L. Ongstad, H.C. Zec, and J.C. Bischof, Biochim. Biophys. Acta —Biomembranes 1768(3), 728–736 (2007).
(76) R. Bhargava, D.C. Fernandez, S.M. Hewitt, and I.W. Levin, Biochim. Biophys. Acta — Biomembranes 1758(7), 830–845 (2006).
(77) P. Lasch, M. Diem, and D. Naumann, in FT-IR Microspectroscopic Imaging of Prostate Tissue Sections, Biomedical Vibrational Spectroscopy and Biohazard Detection Technologies (San Jose, California, 2004; SPIE), pp. 1–9.
(78) R. Bhargava, S.M. Hewitt, and I.W. Levin, Nat. Biotech. 25(1), 31–33 (2007).

AI Shakes Up Spectroscopy as New Tools Reveal the Secret Life of Molecules
April 14th 2025A leading-edge review led by researchers at Oak Ridge National Laboratory and MIT explores how artificial intelligence is revolutionizing the study of molecular vibrations and phonon dynamics. From infrared and Raman spectroscopy to neutron and X-ray scattering, AI is transforming how scientists interpret vibrational spectra and predict material behaviors.
Real-Time Battery Health Tracking Using Fiber-Optic Sensors
April 9th 2025A new study by researchers from Palo Alto Research Center (PARC, a Xerox Company) and LG Chem Power presents a novel method for real-time battery monitoring using embedded fiber-optic sensors. This approach enhances state-of-charge (SOC) and state-of-health (SOH) estimations, potentially improving the efficiency and lifespan of lithium-ion batteries in electric vehicles (xEVs).
New Study Provides Insights into Chiral Smectic Phases
March 31st 2025Researchers from the Institute of Nuclear Physics Polish Academy of Sciences have unveiled new insights into the molecular arrangement of the 7HH6 compound’s smectic phases using X-ray diffraction (XRD) and infrared (IR) spectroscopy.