ICP-MS: Key Steps to Control Contamination and Achieve Low Detection Limits
Inductively coupled plasma mass spectrometry (ICP-MS) instruments can perform low-level elemental analysis in a wide range of sample types, from high-purity chemicals to high matrix digests. But achieving consistently low detection limits requires good control of elemental contamination, as well as spectral interferences. A clean working area, careful selection of reagents, and good sample handling techniques are key to successful trace and ultratrace elemental analysis. In this article, we provide five practical tips for controlling contaminants and minimizing detection limits.
Inductively coupled plasma-mass spectrometry (ICP-MS) instruments offer high sensitivity and low random background, which enables the technique to provide ng/L (ppt) level trace element analysis. But routinely achieving low detection limits requires more than just a sensitive instrument. In industries such as semiconductor manufacturing, experienced analysts develop working practices that minimize the potential for contamination to affect results. The following practical suggestions can be implemented in any laboratory, and will help ensure data quality is not compromised by contamination that could have easily been prevented.
Consider Whether the Laboratory Environment is Suitable for Your Analysis
Analytical laboratories doing trace element analysis in industries such as environmental monitoring, food safety, pharmaceutical manufacturing, and clinical research do not need a special clean laboratory environment to achieve the method detection limits (DLs). But even a normal trace element laboratory should be designed and constructed in a way that minimizes sources of elemental contamination.
In the semiconductor manufacturing and process chemical industries, trace elements are routinely measured at ppt and sub-ppt levels, so laboratories in these industries often install a dedicated cleanroom. Cleanrooms are classified according to the number of particles of various sizes between 0.1 and 5 microns per unit volume of laboratory air. For example, an International Organization for Standardization (ISO) Class 3 laboratory (equivalent to the earlier US Federal Standard 209E [FS209E] Class 1) can contain a maximum of 8 particles of 1 micron and 1000 particles of 0.1 micron per cubic meter of air. An ISO Class 7 laboratory (FS209E Class 10,000) may contain up to 83,200 particles of 1 micron per cubic meter.
However, ISO Class 1 to 4 cleanrooms are expensive to build and maintain, and only retain their rated classification through strict control of access and working practices. A lower cost alternative is to install the ICP-MS in a small Class 10 enclosure located inside a Class 10,000 laboratory or normal trace element laboratory. Another option is to place just the autosampler in a clean enclosure, such as a high efficiency particulate air (HEPA) filtered laminar flow hood. Preparation of samples and standards can also be performed in a similar hood to avoid contamination of vials and solutions while they are open on the bench.
Limit Sources of Particulate Contamination
In a typical laboratory, one of the most problematic sources of sample contamination is airborne particulate material. Consider where contamination may come from and try to eliminate particulate sources from the laboratory.
Common sources of particles in the laboratory include air conditioning units (especially those with overhead vents), corroded bare metal surfaces, printers and personal computers (PCs), and recirculating water chillers, particularly where a fan is used in an air-cooled heat exchanger. Contamination can also arise from dirt and dust brought into the laboratory on people’s shoes, clothing, and personal belongings. Simple steps can remove or reduce the contribution from these sources; for example, by placing the water recirculator in an adjacent service room, or printing to a remote device in a neighboring office. Sticky mats by entrance doors can significantly reduce the amount of dust brought into the laboratory on people’s shoes.
Using appropriate materials during laboratory-based activities can also help to reduce contamination. Gloves are routinely used for safety reasons when handling potentially corrosive acids, and powder free nitrile gloves will help minimize particle contamination.
With a little thought and attention to the way laboratory services, equipment, and personnel are managed, particulate contamination in the laboratory can be minimized without the high costs of installing and operating a dedicated cleanroom.
Avoid Glass! Use Plastic Labware to Minimize Elemental Backgrounds
The following recommendations are appropriate for laboratories that analyze typical ICP-MS sample types prepared and stabilized in dilute acid solutions. If other solvents are used, the details of the cleaning and rinsing protocols will need to be adjusted as appropriate.
Sample vials, pipette tips, and other labware that comes into contact with the sample solution can be a significant source of contamination. Acidic solutions should not be prepared or stored in glassware, even if it has been precleaned, as the acid will extract metal contaminants from the glass. Alkaline solvents such as ammonium hydroxide and tetramethylammonium hydroxide (TMAH) will also extract metals from glass containers. Plastic labware is much cleaner than glass, but brands that contain pigments made with metal additives should be avoided where possible. Clear plasticware made of materials such as polypropylene (PP), low density polyethylene (LDPE), polyethylene terephthalate (PET), or fluoropolymers (PTFE, FEP and PFA) are recommended for the best chemical resistance and lowest levels of contamination. New labware should be acid rinsed to remove surface contamination and manufacturing residues prior to use.
Polypropylene sample vials and centrifuge tubes (15 mL or 50 mL) from brands such as DigiTUBE, Corning, or Nalgene are inexpensive, largely free from metal contamination and are Class A graduated so can be used for volumetric sample preparation. Many laboratories prepare standards and do sample dilution by weight. Conical base DigiTUBEs are available with a skirt to allow them to stand upright on the balance or bench, without needing to be held in an autosampler rack.
Implement Effective Procedures for Cleaning, Storage, and Use of Labware
If the laboratory’s workflow and sample throughput allow, it is good practice to soak sample vials and tubes before use in a covered, clear plastic tank containing ultrapure water (UPW) or dilute acid (for example 0.1% HNO3), as shown in Figure 1. These plastic containers can be purchased at relatively low cost from a normal hardware store. This sort of precleaning approach will help remove manufacturing residues such as mold release agents, which can contain metals, including Al and Zn. More aggressive cleaning may be required to achieve the lowest background levels for ultratrace analysis. Vials and containers should be rinsed three times in UPW prior to use.
Figure 1: Clear plastic containers of various sizes used for cleaning and storage of sample tubes and other consumables.

The same sort of precleaning approach can also be used for soaking pipette tips before use, although clear tips supplied in a covered container will be dust free and do not usually require precleaning except for when ultralow level analysis is required. The rinse baths should not become significantly contaminated, and can usually be used for a year or more before the UPW or acid needs to be changed.
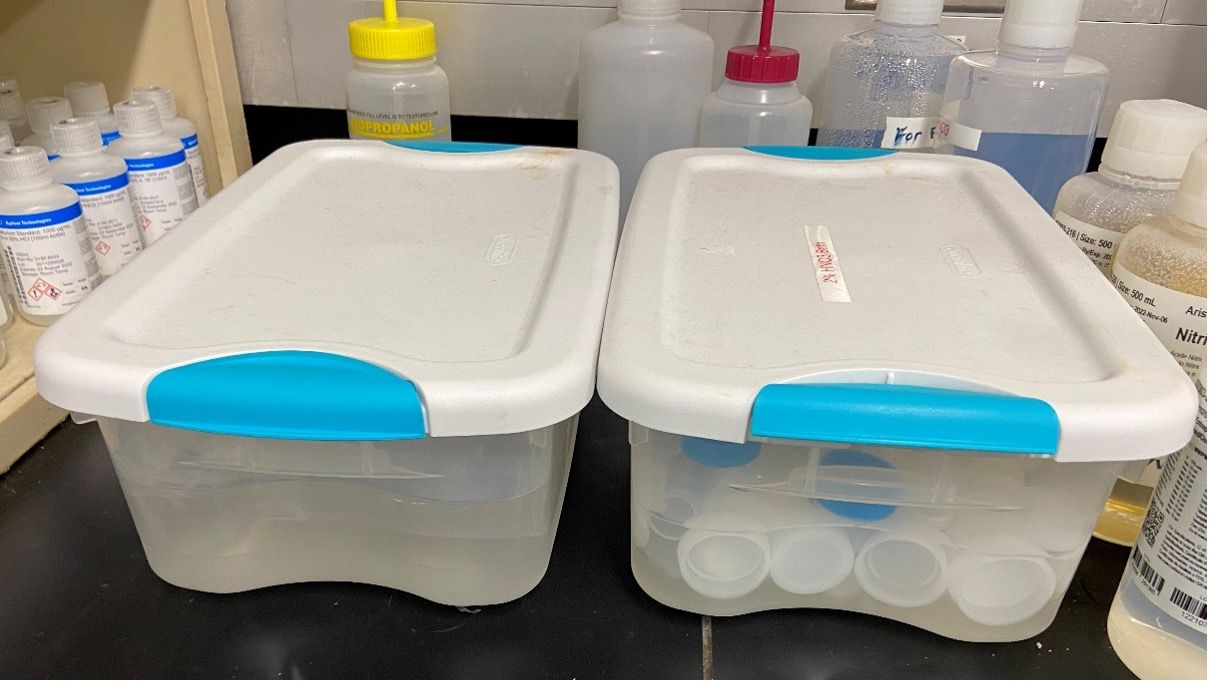
Similar clear plastic storage containers can be used to clean and store ICP-MS sample introduction components, ideally with separate containers for different components, such as standard and inert kits (Figure 2). Sample introduction parts such as the quartz spray chamber and torch can be soaked in an acid bath, then dried before use. Interface cones can be sonicated in UPW or a dilute laboratory cleaning agent such as Citranox, then dried and stored in a sealed container. For very contaminated cones, a fine abrasive polishing powder and a pointed Q-tip can be used, followed by sonication in UPW.
Figure 2: ICP-MS sample introduction components are cleaned and stored in separate containers for different kits, for example standard quartz and PFA inert kit components.
Use Reagents of Appropriate Quality for the Analysis
Good quality deionized water (18 MΩ.cm) is essential to maintain the low background levels required for trace level analysis, particularly for common contaminants such as Na, Al, and Fe. The elements B and Si are more difficult for ion exchange systems to remove, so pay particular attention to the background levels for these elements. An increase in B or Si backgrounds may indicate that the ion exchange cartridge of your ultrapure water system needs to be replaced.
The quality of reagents such as acids should be appropriate for the levels of trace elements being measured but, in general, using better quality acids will reduce the time you have to spend dealing with errors due to contamination. A viable alternative to high purity acids is to purchase reagent grade acids and purify them in the laboratory using sub-boiling distillation.
When using concentrated high purity acids to prepare samples, calibration standards, and quality control standards (QCS), a small volume of acid should be decanted into a micro beaker or the acid bottle lid before pipetting into the sample or standard vials. This will help avoid contaminating the acid in the bottle.
Once empty, the perfluoroalkoxy alkanes (PFA) bottles that high purity acids are supplied in can be rinsed and dried and will then be excellent metal-free containers for subsequent use. These bottles can be used to prepare and store diluents, and can even be used for low-level standard addition analysis with the spikes being added directly into the sample in the reused acid bottle.
Summary
These practical tips from experienced ICP-MS analysts have been demonstrated to reduce errors due to contamination. By implementing a few simple procedures and ensuring reagents and consumables are suitable for the intended analysis, ICP-MS users can minimize the possibility of data quality being compromised by trace element contamination.
Bert Woods and Ed McCurdy are with Agilent Technologies, Inc. Direct correspondence to: ed_mccurdy@agilent.com

FT-IR Microscopy, Part 2: Mid-IR Sampling with DRIFTS, IRRAS, and ATR
February 14th 2025Fourier transform infrared (FT-IR) microscopy using reflection methods (diffuse reflection, reflection/reflection-absorption, or attenuated total reflectance) typically requires less sample preparation than transmission. However, optimal results will depend upon the sample and, in particular, the sample surface.
Trending on Spectroscopy: The Top Content of 2024
December 30th 2024In 2024, we launched multiple content series, covered major conferences, presented two awards, and continued our monthly Analytically Speaking episodes. Below, you'll find a selection of the most popular content from Spectroscopy over the past year.