Key Steps to Follow in a FRET Experiment
Förster resonance energy transfer (FRET) is a versatile part of the toolbox of fluorescence methods. This through-space, photon-less energy transfer process between a donor fluorophore and an acceptor chromophore is perhaps most famous for its utility as a “molecular ruler” that can resolve nanometer-scale distances. FRET is also a popular and advantageous basis for biomolecular assays and sensors.
One of the most attractive features of the Förster resonance energy transfer (FRET) mechanism is that it can be measured with the simplest of fluorimeters up through to the most advanced single-molecule fluorescence systems. However, this relative simplicity sometimes belies careful considerations that must be taken into account in most FRET experiments. As the user base for FRET and the scope of its applications continues to grow, it is important to understand how to design an optimized and rigorous FRET experiment. This article provides a brief overview of the key considerations, most of which are linked to Figure 1. A much more detailed and broader scope of guidance can be found in the literature (1), and readers are encouraged to review the fundamental FRET theory in the literature (2,3).
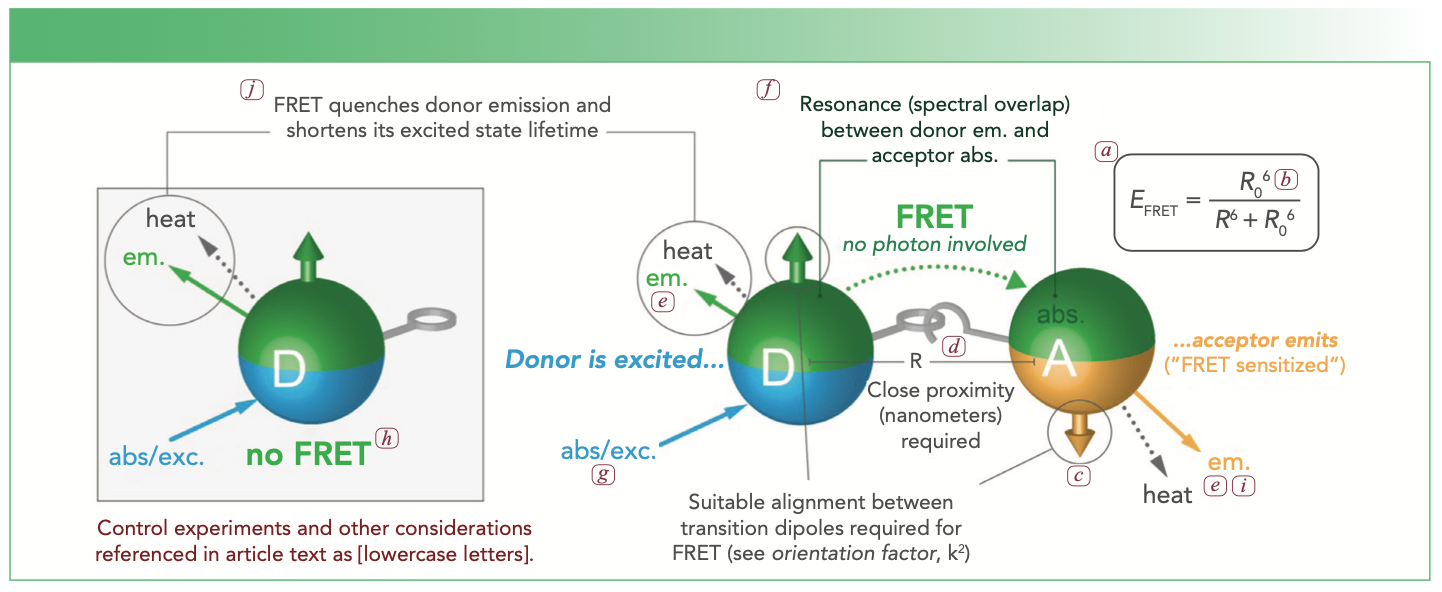
FIGURE 1: Conceptual illustration of FRET. Parts of this illustration are linked to the discussion in the text through lowercase letter labels (a–j).
Know the Limits of Common Theory
Although basic FRET theory can be found in numerous publications and on a multitude of websites, these basics are not always sufficient. For example, the classic equation (shown in Figure 1a) for FRET efficiency (the probability of FRET occurring) applies to a uniform ensemble of discrete one donor–one acceptor pairs. This equation must be modified if multiple acceptors interact with a common donor, and will often be a poor approximation if multiple donors interact with a common acceptor, if a system is highly heterogeneous in its donor–acceptor combinations, or if different FRET pathways compete with one another. Such non-classic systems will need to be modeled using analyses that compare the rates of the various FRET pathways against one another and the rates of other excitation and relaxation processes (4).
Furthermore, many sources present the equation for the FRET-relevant spectral overlap integral (between donor emission and acceptor absorption) without specifying units for the quantities, which must be correct for downstream estimation of the Förster distance, R0 (Figure 1b). Units should thus be confirmed in a reference source or assessed by dimensional analysis. Most estimations of a Förster distance will also assume a “dynamic averaging regime” with an orientation factor of κ2 = 2/3 between donor and acceptor (Figures 1b, 1c). A recent perspective has addressed potential challenges from this assumption in detail (5). Adopting κ2 = 2/3 or another “special” value (such as, for example, static averaging regime) is not necessarily a bad practice, provided the uncertainties associated with this assumption are factored into drawing conclusions from an experiment—a conclusion predicated on an accurate and precise value for κ2 is usually not a solid conclusion.
Define the Mechanism of Donor-Acceptor Proximity
An essential requirement of FRET is close proximity between donor and acceptor, usually within 10 nm, but up to approximately 15 nm in special cases. A rigorous FRET experiment will rationally design or, at minimum, understand the origin of donor-acceptor proximity (Figure 1d). Common approaches include having the donor and the acceptor attached to the same macromolecular scaffold, and also having molecules that are separately labeled with donor and acceptor interacting through specific binding, aggregation, or assembly on a common scaffold or surface.
Match the Measurement Modality and FRET Metric to the Objective and Limitations of the Experiment
FRET is famously useful as a “molecular ruler” because the energy transfer efficiency can be used to measure the nanometric distance between donor and acceptor. For such measurements, it is important that the FRET efficiency has maximum variation around the distance of interest. This means avoiding too close an approach to the plateaus in FRET efficiency near 0 and 100%. A mathematical model of FRET efficiency versus distance for a hypothetical system will indicate a range over which efficiency is approximately linear with distance, although the overall relation is sigmoidal.
FRET is also a useful mechanism for sensors. For this application, it is often preferred that the FRET efficiency switches between a negligible value (~0%) and a significant value upon detection of the target analyte (or otherwise maximizes the relative change in the measured FRET signal). This signal change also has a role in determining the concentration of FRET probe that should be used. For example, if it is possible to detect the switch in FRET efficiency for one probe out of 100, then the concentration of probe should not exceed 100-fold of the lowest concentration of target analyte to be detected.
A FRET measurement must also be designed around the available technical capabilities. Important considerations are as follows: whether the metric for assessing FRET will be based on fluorescence intensity or lifetime; whether this metric will be measured for the donor, the acceptor, or both (as applicable); and whether resolution of donor and acceptor emission will be via emission filters or spectrally via monochromator (Figure 1e). For the latter, spectral measurements allow more robust correction of overlapping donor and acceptor fluorescence emission and may thus accommodate a wider array of potential donor–acceptor pairs. Crosstalk between detection channels (wavelengths or filters) for donor and acceptor emission is common and should be checked for, and may require mathematical correction to avoid skewing the value of the chosen FRET metric.
Ensemble FRET efficiency is best reserved as a metric for homogeneous systems. The apparent ensemble efficiency for heterogeneous systems is a function of both the real FRET efficiencies and fractions of whatever subpopulations are in the system. Thus, the ensemble efficiency for a heterogeneous system loses meaning without independent knowledge of the sub-populations. For example, sensing applications detect initially unknown concentrations of the analyte and are better suited to a metric such as donor intensity or lifetime, acceptor intensity, or an acceptor–donor intensity ratio.
Donor and acceptor fluorescence intensities are simple and versatile metrics, but have the drawbacks of being sensitive to the concentration of the FRET pair, measurement volume, and drift or fluctuation in instrumentation. The acceptor–donor intensity ratio and donor fluorescence lifetime are more robust metrics because they are approximately insensitive to sample volume and concentration over whatever range the donor–acceptor proximity is unaffected by concentration. An acceptor–donor intensity ratio and donor fluorescence lifetime are also either self-correcting or less affected by many forms of instrumental drift or fluctuation. Many interesting applications of FRET have a biological system as the gatekeeper of a number of FRET probes being measured (not well-controlled by the experimentalist), making this advantage significant.
Another consideration is whether an ensemble measurement is satisfactory or if single-molecule measurements are necessary. Single-molecule measurements are most informative when heterogeneity or asynchronous processes within a sample are of interest, but are beyond the scope of this short article.
Choose an Optimum Donor–Acceptor Pair
The first and best-known criterion for a good donor–acceptor pair is substantial overlap between the donor emission spectrum and the acceptor absorption spectrum (Figure 1f). However, the considerations do not stop here. The magnitude of the Förster distance must be commensurate with the experimental objective and the mechanism of donor–acceptor proximity. Moreover, the labeling chemistries, solubility, charges, and sizes of the donor and acceptor should also be good matches to the mechanism of proximity. For a FRET pair where both donor and acceptor are fluorescent, it is also preferable that there is an excitation wavelength for the donor at which direct excitation of the acceptor is negligible (Figure 1g). The choices of donor and acceptor must also align with available technical capabilities (such as satisfactory excitation wavelengths and emission filters, suitable resistance to photobleaching). For all of the foregoing, there are pros and cons for the many candidate donor and acceptor materials available, which range from organic dyes to fluorescent proteins to various nanoparticles. One should go into a FRET experiment anticipating that multiple FRET pairs may need to be tried to achieve optimum results.
Implement Control Samples
Some general challenges with fluorescence include the potential sensitivity of the fluorescence intensity of a dye to its local microenvironment (such as viscosity or other restriction of movement, solute concentrations, and more), the lack of a standardized unit for fluorescence intensity, and potentially large differences in brightness between different fluorophores. Consequently, control experiments that ensure that changes in donor and acceptor emission arise from FRET and not from other processes are essential. With only a few exceptions, experimental samples and control samples must usually be measured under the same instrument and with the same settings.
The minimum control sample is a no-FRET reference state (Figure 1h) with only the donor, but more control samples would be beneficial. For example, reabsorption of the donor emission by an acceptor could give the appearance of donor quenching and acceptor sensitization, but is not actually FRET. A control experiment that has a combination of donor and acceptor but with disrupted proximity (Figure 1d) will guard against such trivial energy transfer via reabsorption. If the mechanism of proximity is sufficiently robust, consistent FRET metrics over a range of dilutions may also be a guard against trivial energy transfer.
For a fluorescent acceptor, another important control is a sample with only an acceptor to distinguish between FRET-sensitized acceptor emission and directly excited acceptor emission (Figure 1i). Potential brightness differences mean that the latter cannot be reliably assumed to be negligible simply from qualitative inspection of an absorption spectrum.
It is also good practice to ensure that two metrics yield similar FRET efficiencies for samples expected to have maximum FRET. Commonly, the decreases in donor intensity and lifetime (relative to a no-FRET reference state) are compared (Figure 1j). Commensurate values will tend to indicate an absence of trivial energy transfer. Even when not the preferred metric for all the experiments, a one-time measurement of lifetime may help to confirm FRET. Alternatively (or in addition), quantitative comparison of a measured FRET metric to a predicted one can be diagnostic, as a large discrepancy may indicate something other than FRET (or a poor model of an actual FRET system).
Summary
Ensemble FRET measurements are both powerful and accessible to a broad array of experimentalists. Although it surprisingly easy to be fooled into a false observation of FRET, it does not take much additional effort or resources to safeguard against false observations. Again, this article is intended to be a starting point—there are certainly details and special cases beyond the scope of this text. Nevertheless, the above guidance is a solid foundation for many applications of FRET.
References
(1) W.R. Algar, N. Hildebrandt, S.S. Vogel, and I.L. Medintz, Nat. Meth. 16, 815–829 (2019).
(2) N. Hildebrandt, C.M. Spillmann, W.R. Algar, T. Pons, M.H. Stewart, E. Oh, K. Susumu, et al, Chem. Rev. 117, 536–711 (2017).
(3) B.W. van der Meer, in FRET – Förster Resonance Energy Transfer, I.L. Medintz and N. Hildebrandt, Eds. (Wiley-VCH Verlag GmbH & Co., Weinheim, Germany, 2013), pp. 23–62.
(4) M. Massey, H. Kim, E.M. Conroy, and W.R. Algar, J. Phys. Chem. C 121, 13345–13356 (2017).
(5) B.W. van der Meer, Meth. Appl. Fluoresc. 8, 030401 (2020).
W. Russ Algar is with the Department of Chemistry at the University of British Columbia, in Vancouver, British Columbia, Canada. Direct correspondence to: algar@chem.ubc.ca ●

New Study Reveals Insights into Phenol’s Behavior in Ice
April 16th 2025A new study published in Spectrochimica Acta Part A by Dominik Heger and colleagues at Masaryk University reveals that phenol's photophysical properties change significantly when frozen, potentially enabling its breakdown by sunlight in icy environments.
Tracking Molecular Transport in Chromatographic Particles with Single-Molecule Fluorescence Imaging
May 18th 2012An interview with Justin Cooper, winner of a 2011 FACSS Innovation Award. Part of a new podcast series presented in collaboration with the Federation of Analytical Chemistry and Spectroscopy Societies (FACSS), in connection with SciX 2012 ? the Great Scientific Exchange, the North American conference (39th Annual) of FACSS.
Can Fluorescence Spectroscopy Evaluate Soil Dissolved Organic Matter Dynamics?
February 20th 2025A new study published in Chemical Engineering Journal by researchers from Northeast Agricultural University in China reveals that biochar aging, influenced by environmental factors like UV exposure and wet-dry cycles, alters dissolved organic matter composition and affects its effectiveness in remediating cadmium-contaminated soil.
FT-IR Microscopy, Part 2: Mid-IR Sampling with DRIFTS, IRRAS, and ATR
February 14th 2025Fourier transform infrared (FT-IR) microscopy using reflection methods (diffuse reflection, reflection/reflection-absorption, or attenuated total reflectance) typically requires less sample preparation than transmission. However, optimal results will depend upon the sample and, in particular, the sample surface.
New Fluorescent Raman Technique Enhances Detection of Microplastics in Seawater
November 19th 2024A novel method using fluorescence labeling and differential Raman spectroscopy claims to offer a more efficient, accurate approach to detect microplastics in seawater. Developed by researchers at the Ocean University of China, this method improves both the speed and precision of microplastic identification, addressing a key environmental issue affecting marine ecosystems.