- October 2022
- Volume 37
- Issue 10
- Pages: 25–28
Infrared Spectroscopy of Polymers, VIII: Polyesters and the Rule of Three
An exploration of polyethylene terephthalate (PET), one of the most important polymers, is presented.
In the last column, we reviewed the spectroscopy of the carbonyl group and that of ketones. We then analyzed our first C=O containing polymer. In this column, we continue to study C=O containing polymers by first reviewing the spectroscopy of the ester functional group and then analyzing the spectra of several important polyesters. Among the spectra we study is that of polyethylene terephthalate (PET), one of the most economically important polymers in the world.
Recall (1) that if an alcohol and a carboxylic acid are reacted, a functional group called an ester is synthesized, and the reaction is called esterification. The molecular framework of the ester functional group is shown in Figure 1.
In the figure, the carbon in the C=O group is called the carbonyl carbon, the carbon to the left of the carbonyl carbon is called the alpha carbon, and the oxygen to the right is called the ester oxygen. If the alpha carbon is saturated, it gives a saturated ester, whereas if the alpha carbon is aromatic, we have an aromatic ester. Esters are commonly found in food and generally taste good. For example, isoamyl acetate is what gives a banana some of its flavor, and ethyl acetate gives cherries some of their flavor. Polyesters are important polymeric materials being made into beverage bottles, fabric, and clothing and hence are worth studying.
The Infrared (IR) Spectroscopy of Esters: A Review
Note from Figure 1 that ester groups contain a carbonyl bond and two C-O bonds. We have already studied the IR spectroscopy of these bonds, so we can take a stab at predicting what their spectra might look like. Recall (2–7) that carbonyl stretching peaks are strong and generally occur between 1800 and 1600 cm-1 (assume all peak positions noted in this article will be in cm-1 units, even if not explicitly stated). We also know (6) that C-O stretches are intense peaks typically seen between 1300 and 1000. Because one of the C-O bonds in the ester group is attached to the carbonyl carbon and the other is not, we might expect them to be chemically distinct, have different force constants, and hence give rise to two separate peaks between 1300 and 1000.
As it turns out, our predictions are correct. Esters have a memorable pattern of three intense peaks at ~1700, ~1200, and ~1100 from the C=O and two C-O stretches, and hence follow what I call the “Rule of Three” (1). The first of these peaks is the C=O stretch, which we know from previous experience (7) will be an intense peak in the vicinity of 1700. We also learned last time that carbonyl stretches for aromatic carbonyl groups tend to be 30 cm-1 lower than for saturated carbonyl groups because of conjugation (7). Thus, for saturated esters, the C=O stretch falls from 1755 to 1735. Note that this is a little higher than some other saturated C=O groups, and if you see a C=O stretch around 1750, one of your first thoughts should be saturated ester.
For aromatic esters, the C=O stretch falls from 1730 to 1715. This is a tricky peak position because several other functional groups, including saturated ketones (7), have C=O stretching peak positions in this range. This is why, as is typical for carbonyl spectra, we will be dependent upon other peaks besides the C=O stretch to identify the carbonyl functional group present in a sample.
The first C-O single bond stretch of esters and the second of the Rule of Three peaks involves the asymmetric stretching of the alpha carbon, carbonyl carbon, and carbonyl oxygen as seen in Figure 2. I call this the “ester C-C-O stretch (8).”
This vibration is reminiscent of the C-C-C stretch of ketones (7), except that, of course, esters contain a C-C-O linkage instead of a C-C-C linkage. In general, for saturated esters, this peak falls from 1210 to 1160, whereas for aromatic esters, it falls from 1310 to 1250.
The third of our rule of three peaks involves the asymmetric stretch of the ester oxygen and the one or two carbons attached to its right. This is called the “O-C-C stretch (8),” as illustrated in Figure 3.
For saturated esters in general, the O-C-C stretch appears from 1100 to 1030, and from 1130 to 1100 for aromatic esters. A summary of the group wavenumbers for saturated esters is found in Table I.
Note in Table I that there are six separate wavenumber ranges listed because each type of ester, saturated and aromatic, follows its own rule of three. This is because the peak position of all three ester peaks are sensitive as to whether the ester is saturated or aromatic. This means that any of the ester Rule of Three peaks can be used to distinguish saturated from aromatic esters, and, if a sample contains both a saturated and aromatic ester, it will have six ester peaks total.
The Infrared Spectroscopy of Polyesters
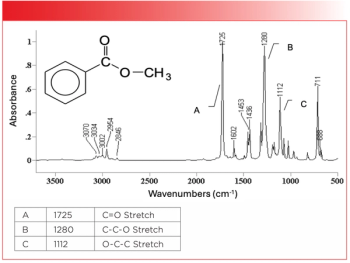
A polyester is a polymer where the ester group is found in the backbone of the polymer chain. A ubiquitous polymer in our modern society is polyethylene terephthalate (PET), otherwise known as soda bottles and polyester clothing. The chemical structure and spectrum of PET are seen in Figure 4.
Note that the ester group is in the backbone of PET, and that it is also an aromatic ester. The Rule of Three peaks in Figure 4 are labeled A, B, and C. Note how these three peaks stick up out of the spectrum like the index, middle, and ring fingers of your hand, and these peaks are often times the three most intense peaks in the spectrum of an ester. In the spectrum of PET, the C=O stretch is at 1721, the C-C-O stretch is at 1245, and the O-C-C stretch is at 1100.
The spectrum of another polyester, polybutylene terephthalate (PET), is seen in Figure 5. The Rule of Three peaks are labeled A, B, and C. The C=O stretch for this polymer is seen at 1730, the C-C-O stretch is at 1286, and the O-C-C stretch falls at 1133. Note that the third of the Rule of Three peaks is smaller than the other two as expected.
The only chemical difference between PET and polybutylene terephthalate is that the former has two methylene groups in a row, whereas the latter has four CH2s in a row. Recall (9) that when an alkyl chain has four or more methylenes in a row, the CH2 rocking peak falls around 730. In Figure 5, this peak is found at 742 and labeled D.
A common biodegradable polymer that you might not think of as being a polyester actually is one. Polylactic acid, which is found in sour milk (among other things), does have a saturated ester group in its backbone, as you can see from its structure in Figure 6. This polymer is becoming increasingly important because it is made from readily available biological materials and will biodegrade, alleviating somewhat the worldwide problem of plastic pollution (10). For example, it is made into biodegradable cutlery (10). Polylactic acid is made from the condensation reaction (splitting off of water) of lactic acid. The structure and spectrum of lactic acid are seen in the top of Figure 6, whereas the structure and spectrum of polylactic acid are seen in the bottom of the figure.
Note that lactic acid is a carboxylic acid, whose spectra we have studied previously (11).
Briefly, the spectra of carboxylic acids are characterized by a broad and intense O-H stretching envelope because of hydrogen bonding from 3500 to 2500, a carbonyl stretch around 1700, and a C-O stretch around 1250. These peaks for lactic acid are labeled A, B, and C in Figure 6.
Note in Figure 6 that, upon the reaction of lactic acid to polylactic acid, the broad O-H stretching envelope has disappeared because the OH of the acid has reacted away. Polylactic acid exhibits the classic ester Rule of Three peaks, as you can see in Figure 6. A is the C=O stretch, B is the C-C-O stretch, and C is the O-C-C stretch. Note that these are the three biggest peaks in the spectrum.
Conclusion
Polyesters contain an ester linkage in their backbone. We reviewed the spectra of esters and noted that they follow the Rule of Three, meaning they have a series of strong peaks at ~1700, ~1200, and ~1100. These three peaks are from C=O, C-C-O, and O-C-C stretching, respectively. We also found that saturated and aromatic esters follow their own Rules of Three, meaning that each of the Rule of Three peaks is sensitive as to whether an ester is saturated or aromatic, and any of these peaks can be used to distinguish between the two different types of esters. We then reviewed the spectra of polyethylene terephthalate, PET, and polylactic acid, and found that they all clearly followed the Rule of Three.
References
(1) B.C. Smith, Spectroscopy 33(7), 20–23 (2018).
(2) B.C. Smith, Spectroscopy 32(9),31–36 (2017).
(3) B.C. Smith, Spectroscopy 32(10), 28–34 (2017).
(4) B.C. Smith, Spectroscopy 33(1), 14–20, (2018).
(5) B.C. Smith, Spectroscopy 33(3), 16–20 (2018).
(6) B.C. Smith, Spectroscopy 33(5), 20–23 (2018).
(7) B.C. Smith, Spectroscopy 37(8), 10–13 (2022).
(8) B.C. Smith, Infrared Spectral Interpretation: A Systematic Approach (CRC Press, Boca Raton, FL, 1999).
(9) B.C. Smith, Spectroscopy 30(7), 26–31, 48 (2015)
(10) “Polylactic acid,” Wikipedia.
(11) B.C. Smith, Spectroscopy 33(1), 14–20 (2018).
Articles in this issue
about 3 years ago
Decimal Versus Binary Representation of Numbers in Computersabout 3 years ago
Where Perception Meets Reality: The Science of Measuring ColorNewsletter
Get essential updates on the latest spectroscopy technologies, regulatory standards, and best practices—subscribe today to Spectroscopy.