Ion Mobility-Mass Spectrometry: A Tool for Characterizing the Petroleome
Spectroscopy
The authors demonstrate the capacity to separate petroleum-derived molecules having the same nominal mass in the mobility dimension using IM-MS spectrometry.

A two-dimensional gas-phase separation technique, ion mobility-mass spectrometry (IM-MS) is applied to the analysis of crude oils. In this approach, ions are generated by laser desorption/ionization and separated before MS analysis on the basis of collision cross-section (that is, apparent surface area of the gas-phase ion). The capacity to separate petroleum-derived molecules having the same nominal mass in the mobility dimension is demonstrated. Inspection of IM-MS spectra reveals a high degree of diversity in structure and chemical class for crude oil components.
Understanding the molecular composition of a crude oil is an essential component of the refining process (1) and a prerequisite for development of new and more efficient processes to convert the full mass balance of a crude into useful energy. Resolving petroleum samples to their molecular composition is a great challenge to analytical and petroleum chemists because of the complex nature of the samples (2). Moreover, petroleum samples are typically composed of thousands (~1000–100,000) of individual molecules (1–3) derived from highly diverse populations of compound types, (such as porphyrins, paraffins, multi-ring aromatics, alkanes, and alkenes) (with varying degrees of branching), and other S-, N-, and O-containing hydrocarbons (2–10). Numerous approaches have been utilized to fully characterize crude oils, but no single technique is fully descriptive, and analyses by different techniques frequently generate conflicting data for the same sample. As a result, complete description of a crude oil is often difficult, if not impossible.
Ultrahigh resolution mass spectrometry (MS) has shown potential to resolve components in a crude oil (1,11,12), but the practical limit is imposed by the rapid increase in the number of possible elemental formulas (with increasing mass), making it difficult to assign unique elemental formulas for ions greater than 300 m/z (1). In the current work, we investigate the potential for ion mobility spectroscopy (IMS) to provide complementary information to MS for characterization of petroleum samples. IMS is a postionization separation technique that separates gas-phase ions based on their collision cross-section; we have shown that when coupled with mass (m/z) separation, ion mobility-mass spectrometry (IM-MS) has unique strength in resolving complex mixtures of peptides, carbon clusters, and various polymeric molecules that have significant overlap in their mass spectra (13–16). In particular, IM-MS provides rapid separation of isomers (17), conformers (18), and species of differing chemical class (19) (based on differences in functional groups, polarities, and atomic compositions), which would be advantageous for the rapid characterization of petroleum samples.
Experimental
The principles for IMS separation have been described in detail elsewhere (20,21). Briefly, ions enter a drift cell filled with an inert gas and migrate along the length of the drift cell under the influence of a weak electrostatic field. The ions experience collisions with the neutral drift gas establishing a constant drift velocity that is related to the electric field (E) through the following equation:

where vD is the drift velocity of the ion and K is a mobility constant that is specific to the ion. The mobility of an ion (K) is inversely related to its ion-neutral collision cross-section (the apparent surface area of the ion) and forms the basis for typical IMS separations; that is, a separation based on size, composition, structure, and charge state.
The IM-MS instrument used in these studies was constructed in collaboration with Ionwerks, Inc. (Houston, Texas). A schematic of the instrument is shown in Figure 1. A 355-nm, Nd:YAG laser was utilized to desorb and ionize samples from a stainless steel sample plate. After laser desorption–ionization (LDI), ions are separated in a 15 cm-long drift cell (periodic high-field, low-field regions) maintained at ~3 torr with He gas. Once IMS separation is complete, ions are focused into a narrow beam using a multielement einzel lens and mass analyzed with an orthogonal time-of-flight (o-TOF) mass spectrometer equipped with an electrostatic ion mirror (R ~2500). In all IMMS experiments, the LDI source was maintained at the same pressure and temperature (approximately 297 K) as the drift cell, and typical IMS field strengths were 10–20 V.cm–1 torr–1 The mass spectrometer was externally calibrated using C60 and C70 (Sigma). All spectra were acquired at a laser intensity equivalent to the observed signal threshold.
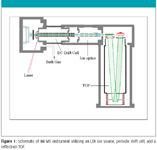
Figure 1
To illustrate the potential of the IM-MS instrumentation, two samples were studied — carbon nanopowder (Sigma), and a Saudi Arabian crude oil (courtesy of Professor Dr. Daulat D. Mamora, Department of Petroleum Eng., TAMU). Samples were used as received without further purification before analysis. LDI-IM-MS analysis was performed by depositing a thin layer of the sample (~1 mL, either in solution or neat) directly onto a stainless steel plate, then inserted into the IM-MS instrument.
Results and Discussion
IMS introduces an additional post-ionization separation before mass analysis, thus providing an inherent increase in the peak capacity of any mass spectrometer (22,23). At the same time, IMS will provide complementary information on the composition and structure of an ion as described earlier. To illustrate the effectiveness of IM-MS in the analysis of hydrocarbon-rich samples, carbon nanopowder is taken as a reference because (i) it provides a high ion yield, and (ii) it produces ions with chemical species ranging from small hydrocarbon clusters to polycyclic–branched aromatic hydrocarbons, to pure carbon clusters. An IM-MS spectrum of carbon nanopowder is shown in Figure 2, which plots the drift time of the ions (arrival time distribution) versus the ion mass (m/z). Notably in the figure, efficient IMS separation is achieved for the single and double charge states of fullerene clusters (inset, bottom right). On close inspection of the lower mass range of Figure 2 (inset, top left), it is evident that additional components that overlap in m/z are resolved in the IMS dimension. These components have been identified as various classes of hydrocarbon clusters that are either present in the sample or formed in the sample when irradiated with photons from the laser (24). These different classes of hydrocarbons are easily resolved in the experiment without incorporating complicated and lengthy prefractionation procedures. In fact, IM-MS separations occur on a timescale of milliseconds compared to a timescale of minutes to hours for traditional approaches such as liquid (4) or gas chromatography (9,25).

Figure 2
The driving force behind the observed IMS separation in Figure 2 can be realized considering the inherent differences in the gas-phase structure: ions that are more compact, branched, or condensed in structure will have shorter arrival time distributions than the more extended and less compact ions. Similarly, ions with similar structure, or of the same molecular class from different samples, will occupy the same space in the 2D plot as the trendlines illustrated in Figure 2, thus enabling their identification based on the correlation between the apparent gas-phase surface area and m/z for a specific compound class.
Analysis of untreated crude oils generally provides complex MS spectra, and information-rich data has been restricted to very high resolution instruments (such as Fourier transform ion cyclotron resonance [FT-ICR]-MS) because of sample complexity in which there are multiple peaks at the same nominal mass. One way to overcome this limitation is by adding a complementary gas-phase separation. For example, Figure 3 contains IMMS spectra of a light crude oil that has not been subjected to pretreatment or prefractionation. The upper portion of the figure (Figure 3a) shows the mass spectra summed over all mobilities and is the MS spectrum that would be obtained if no IMS separation was included. As shown, the molecular weight distribution ranges from 200 to 1100 m/z, peaking at approximately 300 m/z, showing a peak at every nominal mass. When looking closely at the IM-MS data (Figure 3b), it is observed the that the ion signal is spread over a broad range of IMS drift times and includes overlap with both hydrocarbon trendlines obtained from the carbon nanopowder spectra (Figure 2).

Figure 3
Figure 3d contains an expansion of the spectra in Figure 3b, and these data accurately illustrate the structural diversity of the petroleum sample while highlighting the utility of the IM-MS experiment. That is, for a series of ions that increase in mass but maintain similar molecular class, structural features, or conformation (as with the fullerene clusters in Figure 2), an increase in mass is typically accompanied by a near linear increase in arrival time distribution. When viewing the crude oil spectra in two dimensions, it is clear that this trend is not conserved, indicating a high degree of structural, and potentially compositional, diversity for the petroleum sample. It is likely that the relative differences in mobility observed in Figure 3 result directly from the differences in chemical class and type of molecules present in the sample. The utility of analyzing the spectra with two dimensions (mobility and mass) is further highlighted by the 3-D IM-MS spectra projected in Figure 4, in which numerous peaks are observed that coincide in mass (separated by less than a few milli-daltons) but are resolved in the mobility dimension. These data clearly illustrate the advantage of incorporating an IMS separation; that is, multiple, overlapping m/z can be resolved in the mobility dimension, thus greatly simplifying the challenge of petroleum characterization once the observed IMS separations have been related to the inherent nature of the various ions.
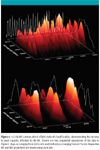
Figure 4
Conclusion
The separation strength offered by IMMS was shown to be highly applicable to petroleum research, where progress is traditionally hindered by sample complexity. Although careful characterization of the space occupied by petroleum samples in the IMMS plots is required at this point, the data herein show that accurate assessment of ion composition and structure from a single IMMS experiment is feasible. Currently, detailed molecular modeling and IMS analysis of model compounds are under way to better understand the behavior of petroleum molecules in the drift cell. Given the current mass resolution of our instrument (~2500), we estimate a three- to fourfold increase in peak capacity when the IMMS spectra are compared with MS-only spectra for analysis of petroleum samples.
Christopher Becker, Francisco A. Fernandez-Lima, and David H. Russell are with the Department of Chemistry, Texas A&M University, College Station, Texas.
References
(1) A.G. Marshall and R.P. Rodgers, Acc. Chem. Res. 37, 53 (2004).
(2) S.K. Panda, J.T. Andersson, and W. Schrader, Anal. Bioanal. Chem. 389, 1329 (2007).
(3) K. Qian, R.P. Rodgers, C.L. Hendrickson, C.L. Emmett, and A.G. Marshall, Energy & Fuels 15, 492 (2001).
(4) M. Ahmaruzzaman and D.K. Sharma, Fuel 87, 1967 (2008).
(5) K.H. Altgelt and M.M. Boduszynski, Energy & Fuels 6, 68 (1992).
(6) M.M. Boduszynski, Energy & Fuels 1, 2 (1987).
(7) M.M. Boduszynski, Energy & Fuels 2, 597 (1988).
(8) M.M. Boduszynski and K.H. Altgelt, Energy & Fuels 6, 72 (1992).
(9) K. Qian, G.J. Dechert, and K.E. Edwards, Int. J. Mass Spectrom. 265, 230 (2007).
(10) R.P. Rodgers, et al., Can. J. Chem. 79, 546 (2001).
(11) C.A. Hughey, R.P. Rodgers, and A.G. Marshall, Anal. Chem. 74, 4145 (2002).
(12) K.E. Qian, J.H. Diehl, and L.A. Green, Energy & Fuels 18, 1784 (2004).
(13) K.J. Gillig, B. Ruotolo, G.F. Verbeck, E. Stone, and D.H. Russell, Rev. Sci. Instrum. (2003).
(14) J.A. McLean, B.T. Ruotolo, K.J. Gillig, and D.H. Russell, Int. J. Mass Spectrom. 240, 301 (Nov. 2004, 2005).
(15) J.A. McLean, J.A. Schultz, M.V. Ugarov, T.F. Egan, and D.H. Russell, Rev. Bras. Apl. Vac. 24, 3 (2005).
(16) B.T. Ruotolo, et al., Anal. Chem. 76, 6727 (Nov. 15, 2004).
(17) A.B. Kanu, P. Dwivedi, M. Tam, L. Matz, and H.H. Hill, J. Mass Spectrom. 43, 1 (2008).
(18) H.A. Sawer, et al., J. Am. Soc. Mass Spectrom. 16, 893 (2005).
(19) B.T. Ruotolo, et al., J. Proteome Res. 1, 303 (2002).
(20) E.A. Mason and E.W. McDaniel, Transport Properties of Ions in Gases (John Wiley & Sons, New York, 1988).
(21) E.W. McDaniel and E. Mason, The Mobility and Diffusion of Ions in Gases (John Wiley & Sons, New York, 1973).
(22) B.T. Ruotolo, J.A. McLean, K.J. Gillig, and D.H. Russell, J. Mass Spectrom. 39, 361 (Apr. 2004).
(23) B.T. Ruotolo, J.A. McLean, K.J. Gillig, and D.H. Russell, J. Am. Soc. Mass Spectom. 16, 158 (Feb. 2005).
(24) C. Becker, K. Qian, and D.H. Russell, Anal. Chem. 80(22), 8592–8597 (2008).
(25) F.C. Wang, K. Qian, and L.A. Green, Anal. Chem. 77, 2777 (2005).

High-Speed Laser MS for Precise, Prep-Free Environmental Particle Tracking
April 21st 2025Scientists at Oak Ridge National Laboratory have demonstrated that a fast, laser-based mass spectrometry method—LA-ICP-TOF-MS—can accurately detect and identify airborne environmental particles, including toxic metal particles like ruthenium, without the need for complex sample preparation. The work offers a breakthrough in rapid, high-resolution analysis of environmental pollutants.
Remembering Engineering Pioneer Sir David McMurtry
December 16th 2024The world of engineering and innovation mourns the loss of a towering figure with the passing of Sir David McMurtry, CBE, RDI, FREng, FRS, CEng, FIMechE, co-founder and Non-Executive Director of Renishaw. Known for his brilliance, humility, and groundbreaking contributions to metrology and manufacturing, McMurtry leaves a legacy that has profoundly shaped modern engineering.
Mass Spectrometry for Forensic Analysis: An Interview with Glen Jackson
November 27th 2024As part of “The Future of Forensic Analysis” content series, Spectroscopy sat down with Glen P. Jackson of West Virginia University to talk about the historical development of mass spectrometry in forensic analysis.
Detecting Cancer Biomarkers in Canines: An Interview with Landulfo Silveira Jr.
November 5th 2024Spectroscopy sat down with Landulfo Silveira Jr. of Universidade Anhembi Morumbi-UAM and Center for Innovation, Technology and Education-CITÉ (São Paulo, Brazil) to talk about his team’s latest research using Raman spectroscopy to detect biomarkers of cancer in canine sera.