LIBS Basics, Part II: Hardware
The choices for LIBS hardware are discussed in detail, particularly lasers and spectrometers, and the trade-offs between cost, size, and performance are illustrated.

Laser-induced breakdown spectroscopy (LIBS) is an emerging analytical method that has been the focus of substantial research over the last 25 years. The recent emergence of commercially available LIBS systems from major manufacturers is a sign of the maturing of the technology. This column installment is the second of a three-part series focusing on major aspects of LIBS. The first installment set the stage with the basics of the LIBS measurement physics and standard applications. This installment discusses the choices for LIBS hardware in detail, particularly lasers and spectrometers, and illustrates the trade-offs between cost, size, and performance. The third installment will discuss LIBS analysis in some depth, exploring the various ways to go from a LIBS spectrum to a solution. Overall, this series is intended to provide an overview for those considering implementation of LIBS to solve a particular analytical problem, and an introduction for those interested in learning more about LIBS.
The growth of interest in laser-induced breakdown spectroscopy (LIBS) analysis of materials has been rapid during the past 10 years, similar to the rise of Raman spectroscopy or atomic emission spectroscopy in earlier decades. Part I of this series (1) documented the nearly exponential rise in LIBS papers from 1995 through 2010. We are now at the point at which many, if not most, people in the analytical community have heard of LIBS, and there are several companies offering commercial LIBS systems that are both laboratory- and industrially focused. The capabilities of the LIBS systems are defined first by their hardware, which defines the laser-sample interaction, the plasma, and the collected spectra, and second by the analytics that interpret the spectra. This installment of "Lasers and Optics Interface" focuses on the hardware portions of LIBS systems, particularly on the laser and spectrometer choices.
Lasers
Three basic parameters are at play in selecting and integrating a laser into a LIBS system: pulse duration, laser wavelength, and laser fluence on the sample. Secondarily, one may consider a double-pulse arrangement in which the ablation laser pulse and analytical pulse are separated by time on the order of microseconds. However, the double-pulse arrangement is typically deployed only in laboratory experiments or very specialized applications because of the added complexity and element- and matrix-specific enhancement observed in the LIBS signal. Because the double-pulse setup is not yet a feature in mainstream systems, we will leave out a detailed discussion of double-pulse interactions and refer readers to one of several excellent reviews available (2).
Nanosecond Versus Femtosecond Lasers
Two basic choices are available for laser pulse duration, nanosecond-class lasers and femtosecond-class lasers. Characteristic lattice vibrations (phonon frequencies) are 1012 –1013 Hz, corresponding at the highest rates to wavelengths on the order of atomic spacing in solids. Laser pulses on the order of a nanosecond (10-9 s) duration are thus very long with respect to characteristic lattice vibration periods in solids, so there is significant heat transfer in the solid during the laser pulse. Picosecond lasers, with pulses on the order of tens or hundreds of picoseconds, are typically in a similar mode of interaction to nanosecond lasers. It is only femtosecond lasers, with pulses typically on the order of 10-13 s, that can interact with solid materials without significant heat transfer.
The implications of the nanosecond versus femtosecond laser choice can be observed pictorially in Figure 1, which shows a laser ablation crater from each class of laser. The nanosecond laser crater (Figure 1a) looks like an impact crater on the moon, with a ridge around the laser impact point and debris scattered around the crater. The crater itself shows evidence of significant melting, and the debris are from direct physical removal and melting as well as nucleation and condensation of small particles from melted vapor in the laser plume. The femtosecond crater (Figure 1b), on the other hand, looks very smooth and appears to have no melting. Femtosecond ablation is much more of a mechanical process, with Coulomb explosion and direct photochemical bond breaking being a few of the major removal processes. Unlike nanosecond ablation spots, there is a minimal heat-affected zone because the process is not thermally driven. As a result, femtosecond ablation produces a more stoichiometric and representative mixture in the plume than does nanosecond laser ablation, because there are no thermal effects.
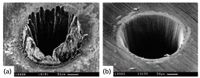
Figure 1: Craters obtained by (a) nanosecond and (b) femtosecond laser ablation. Adapted with permission from reference 3.
For laser ablation inductively coupled plasma–mass spectrometry (ICP-MS) and laser ablation optical emission spectroscopy (OES), femtosecond lasers have been shown to provide more precision and accuracy than nanosecond lasers (4). This is thought to result largely because of the particle distribution from femtosecond ablation, which is markedly smaller (in average size) than the distribution from nanosecond ablation, and thus evaporates better in the plasma torch. However, for LIBS there are countervailing factors in favor of nanosecond lasers for LIBS. The plasma from a typical nanosecond laser is much more robust than a femtosecond pulse. As a result, the integrated emission may be greater, and studies have shown that early in the plasma electron densities and temperatures are higher (5). Overall, the detection limits are comparable for nanosecond and femtosecond LIBS, in most cases, and additional variables such as laser fluence and interactions with the particular solid material being analyzed play a role in the precision and accuracy of the measurement.
Given the considerations in choosing between a nanosecond and a femtosecond laser, cost and reliability typically become the driving factors. Although great strides have been made in femtosecond laser technology in the past decade, femtosecond lasers are still roughly 8–10 times more expensive than comparable nanosecond-class lasers such as Nd:YAG. These lasers cannot be used outside of a laboratory because they are generally more fragile and subject to alignment and reliability issues than nanosecond lasers are. For these reasons, except for high-end laboratory systems that are primarily used in research and development, LIBS systems are typically outfitted with nanosecond-class lasers.
Choice of Laser Wavelength
Given a particular pulse duration for the LIBS system, the laser wavelength also has a large influence on the laser–material interaction that ensues. This influence is more pronounced for nanosecond lasers, because (as previously discussed) the pulse length is long enough to excite phonons in the material and interact with the plasma as the plasma forms. Hence, here I will focus on a few effects that are known to occur for nanosecond lasers. As we will see, the laser wavelength can influence both the ablation and plasma when using a nanosecond laser.
A primary effect in the laser–material interaction is the laser absorption depth, which changes as a function of wavelength. As shown in Figure 2, for some materials such as silicon or germanium, the optical absorption depth at longer wavelengths is measured in tens or hundreds of micrometers or more. Such large characteristic absorption lengths mean that much of the laser pulse is absorbed in the depth of the material. In-depth absorption either causes large and unpredictable ablation, or the energy is deposited at depth and is lost, unable to participate in the ablation and plasma formation process. The rule of thumb that ensues from this consideration is that UV laser wavelengths — for example, 193-nm ArF excimer and 213- and 266-nm Nd:YAG — are required for the application of LIBS to materials that may be optically thin at longer wavelengths. Often, these are materials that appear transparent or translucent to the eye, but many polymers that appear opaque to the eye are still translucent at the fundamental 1064-nm wavelength of the Nd:YAG laser.
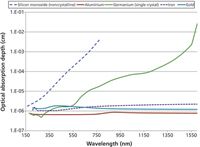
Figure 2: Optical absorption depth as a function of wavelength. Data were obtained and adapted from reference 6.
In general, shorter wavelengths are better for ablation using nanosecond lasers. Shorter wavelengths have smaller absorption depths in essentially all materials, which concentrates ablation energy and promotes efficient ablation. Also, the diffraction-limited spot size is proportional to the wavelength of the laser. As a result, short-wavelength lasers can be focused into smaller spot sizes, which is helpful for microanalysis.
When it comes to plasma formation, however, there are some drawbacks to shorter wavelengths. As the electron density increases in the plasma, there is increased absorption in the plasma because of inverse Bremsstrahlung. The strength of the overall absorption can be described by the interaction of the incoming laser light and the rapid oscillations of electron density in the plasma known as "Langmuir waves." If the laser frequency is lower than the frequency of the Langmuir waves, known as the "plasma frequency," then there is a sharp increase in the absorption in the plasma. Fundamentally speaking, if the electrons are oscillating faster than the laser light, they can keep up with the electromagnetic wave and absorb it. If the laser frequency is higher, there is much lower absorption.
Because the LIBS plasma is forming above a solid sample, the electron density increases from zero to a number on the order of 1017 electrons/cm3. Considering the evolution of the plasma during the 6–8 ns duration of a typical Nd:YAG laser pulse, the beginning portion of the pulse starts the ablation process, ejecting material into a plume and initiating plasma formation through other mechanisms such as multiphoton ionization. The latter portion of the pulse has the opportunity to interact with the expanding plume from the ablation. Depending on the wavelength of the laser, which for example could be 266 nm or 1064 nm for a Nd:YAG laser, the trailing portion of the pulse may be either more absorbed (in the case of a 1064-nm pulse) or less absorbed (in the case of a 266-nm pulse) in the plasma. A longer wavelength (lower frequency) laser will more efficiently couple in to the forming plasma and be absorbed by the plasma at an earlier stage in the plasma lifetime, corresponding with lower electron densities required for absorption. This improved coupling with longer-wavelength lasers results in a more robust plasma, with typically a larger volume and somewhat higher temperature and electron density. Hence, while the shorter-wavelength lasers have an advantage in ablation efficiency and spot size, the longer-wavelength lasers have an advantage in plasma formation.
Laser Energy Influence on the Sample
The total laser energy has an influence on both the material ablation and on the plasma. For a given sample, the amount of energy per unit area (laser fluence) is the relevant important parameter related to energy. It is the fluence that most directly influences the ablation volume. For some samples, increased fluence is too much of a good thing; for example, too much fluence in the analysis of gemstones can cause cracking or unsightly marks. Similarly, pressed pellets or tablets may crack or crumble if the fluence is too high. Therefore, it is not always important to have a high energy, and typically systems have adjustable energy and spot size so that the fluence can be tailored to the material being measured.
Another interesting point is that at any given time the radius of the initial plasma is proportional to energy to the 1/5 power. In particular, R(t) ˜≈ E(1/5).t(2/5), where R is the radius, E is the energy, and t is time. The time, electron density, and temperature (T) progression can be shown to follow a roughly self-similar path (based on hydrodynamic theory) with T(t) ≈˜ E2/3 (7). The upshot of this self-similarity in the decay time of the plasma is that the delay (after the laser) and the gate time (open shutter) of the LIBS detector can be adjusted in predictable ways when the energy is changed in a LIBS experiment.
Detection System
The detection systems used in LIBS can be parameterized by wavelength range, sensitivity, and speed and ability to gate the detector exposure. Overall, the number of choices and range of performance is continually expanding. Given the number of parameters, including cost, that are a consideration in a LIBS system, it's usually true that there is no one-size-fits-all magic bullet that is useful in every situation. Here, we'll cover the main points associated with each of the detector types to provide readers with an overall perspective to make an assessment.
Spectrometer Systems
Charge-Coupled Device–Based Spectrometers
Used in many applications, charge-coupled device (CCD) spectrometers are inexpensive and widely available from many manufacturers. These spectrometers typically use a Czerny-Turner or crossed Czerny-Turner design. To be suitable for LIBS, they must be outfitted with a trigger so that the measurement can be initiated at a known time with no more than a 20–50 ns delay. Presently, none of the CCD systems on the market have a gating feature that allows the detection to be terminated at a known time; these systems are typically measuring for 1 ms or longer following the trigger. Detectors on the standard CCD systems (for example, Toshiba TCD-1304 or Sony ILX511B) are linear arrays and have a typical dynamic range of about 260–300. These chips or similar ones are used in all of the basic CCD systems, and thus all of them have a similar dynamic range. Different manufacturers use various analog-to-digital (A/D) converters with 12-, 14-, and 16-bit ranges, giving the appearance of additional sensitivity, but in reality the higher A/D ranges are simply window dressing and not performance-related. Spectrometers used in the UV region of the spectrum are typically enhanced with a lumogen coating.
Broadband CCD Spectrometers
These spectrometers are arrangements of CCD spectrometers with a common triggering system. Accompanying software can be used to stitch together the output of these systems into a single broadband spectrum. At best, these systems generally provide 0.05 nm full width at half maximum (FWHM) resolution.
Echelle Spectrometers
Echelle spectrometers combine two dispersive elements, typically a prism and a grating, to disperse light in two dimensions on a square detector. This results in a two-dimensional spectral field with various orders in one dimension and wavelength in the other. Software is used to sort the various orders and construct a continuous spectrum, which can range from 200–900 nm or more. Light throughput in these spectrometers is not stellar, typically they have an effective F-number of 7 or 8. Depending on the wavelength of the emission line in question, echelle spectrometers coupled with intensified cameras (see below) can easily outperform CCD spectrometers by an order of magnitude or more. Resolution is typically 0.05 nm or better, but cost, particularly when considering the cost of the camera, can be substantial.
Traditional Czerny-Turner Spectrometers
These more traditional, large Czerny-Turner spectrometers may be 0.25 m in pathlength or more. Typically outfitted with a turret with several diffraction gratings, users can dial the center wavelength, wavelength range, and associated resolution to their liking. The light throughput on these systems is very high and light is generally dispersed using the (very strong) first order of the grating. On a typical intensified camera detector, the light is dispersed across the detector and users can choose to collect ("bin") the camera from one to the maximum number of rows on the camera. Because the gain on the camera can also be adjusted, this combination results in an instrument with incredible dynamic range (104 –105). The downside is that the limitations on the width of the detector result in the collection of only a narrow window of light (30–50 nm) with each shot, if 0.1 nm resolution or better in the spectrum is desired. Hence, associations between multiple elements may be difficult to obtain because the emission lines of the particular elements of interest may be in different parts of the spectrum. This limitation understood, the combination of components is one of the most sensitive LIBS detection schemes, surpassed only by the photomultiplier tube discussed below.
Detectors
CCD Detectors
Inexpensive CCD detectors were covered in some detail above. Beyond these very low-end detectors, there are numerous variations of CCD detectors that are more sensitive than the basic detector. Back-thinned detectors can have enhanced sensitivity, while high-quality CCDs that are cooled with multistage cooling can have signal-to-noise ratios that exceed those of an intensified CCD. The main drawback of these detectors is that the exposure cannot be gated (turned on and off) as can the other detectors. These are available in square (for example, 2048 × 2048) and "spectroscopy" (for example, 1340 × 100) formats.
Intensified CCD Detectors
Intensified charge-coupled devices (ICCDs) have long been the mainstay for LIBS applications. As described above, ICCDs can be gated (some as fast as 2 ns) and have a dynamic range exceeding 104. To accomplish this, the output of a photocathode is amplified (under vacuum) by a microchannel plate amplifier. The resulting electrons strike a phosphor, which is fiber-coupled to a CCD detector. The variable voltage that can be applied to the microchannel plate provides both the high gain potential and the gating capability of the device. Largely because of the uncertainties in the microchannel plate amplifier, ICCDs can be quite noisy at high gain settings. In addition, images are not as sharp as a typical high-quality CCD camera because there can be some "smearing" caused by crosstalk between the fibers coupling the light from the phosphor to the CCD. These cameras are quite expensive.
Electron-Multiplying CCD Detectors
Among the new kids on the block, electron multiplying charge-coupled device (EMCCD) cameras have generated some interest in the LIBS community because of their high sensitivity and much lower cost than ICCDs. In EMCCD cameras, the readout register has multiple preamplification stages before A/D conversion. This allows low-light signals to be enhanced before conversion, thus moving a low-light signal above the A/D noise floor. The gain possible in EMCCDs is about 103. Precision and speed of gating, however, has been a development issue for LIBS. Because of the coupling of the preamplifier and the A/D conversion, the gating has an inherent minimum delay and the actual gate open time may vary across the chip because the chip is read out. Efforts are being made to address these issues to make EMCCDs more on par with ICCD gating performance. EMCCD cameras, as of this writing, are approximately 30–40% the cost of ICCDs, so they are worth watching as they mature.
Photomultiplier Tubes
Photomultiplier tubes (PMTs) are detectors of the last century, correct? For the students reading this, these were the detectors that "old" guys like this author used with a scanning monochromator to obtain broadband emission spectra in 20 min flat. (Yes, one spectrum in 20 min. Today's students are spoiled!) Not so fast. With gains of up to 107 and fast readout electronics available, in some situations they may be the most versatile detectors for LIBS. They can be used for either emission-line-selective filters or in a spectrometer configuration in conjunction with a Paschen-Runge spectrometer. Don't count these detectors out for the ultimate in speed (kilohertz acquisition possible) and sensitivity.
Figure 3 summarizes the salient features of detection systems. Spectrometers or detectors are graded on a relative scale for broadband nature, sensitivity, triggering and gating, and cost. It is obvious, as we stated at the outset, that there are few clear winners. The one statement that can be made with confidence is that if one has a single, known emission line to measure, a PMT system is worthy of consideration. Presently, many OES systems still use PMT arrays for line detection for this reason.
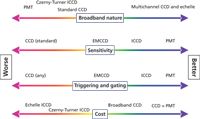
Figure 3: Relative performance of detection system components.
Summary
This installment, part II in a three-part series, was intended to dissect the basic technology choices for components of LIBS hardware. There is not a perfect solution for all situations by any means, but there are excellent, and always improving, options for these components. We hope that this provides a basis to compare and contrast the laser, spectrometer, and detector choices such that when you are seeking a LIBS system solution from one of the manufacturers, you have a road map in mind. The last installment of this series will cover LIBS spectral analysis and the basics of proceeding from a spectrum to a solution. Stay tuned!
References
(1) S. Buckley, Spectroscopy29(1), 22–29 (2014).
(2) J. Scaffaldi, S.M. Angel, and D. Cremers, Anal. Chem.78, 24–32 (2006).
(3) R. LeHarzic et al., Appl. Phys. Lett.80, 3886 (2002).
(4) Z. Yang, B. Fryer, H. Longerich, J. Gagnon, and I. Samson, J. Anal. At. Spectr.26(2), 341–351 (2011).
(5) A. Elhassan, A. Giakoumaki, D. Anglos, G. Ingo, L. Robbiola, and M. Harith, Spectrochim. Acta, Part B 63, 504–511 (2008).
(6) D. Lide, CRC Handbook of Chemistry and Physics (88th ed.) (CRC, Boca Raton, Florida, 2007).
(7) Y. Zel'dovich and Y. Raizer, Physics of Shock Waves and High-Temperature Hydrodynamic Phenomena (Dover, Mineola, New York, 2002).
Steve Buckley is a Director of Market Development at TSI Incorporated, which acquired the LIBS business of Photon Machines, Inc., in 2012. Before cofounding Photon Machines, Steve was a tenured professor of engineering at the University of California at San Diego. He has been working on research and practical issues surrounding the implementation of LIBS since 1998. He can be reached at steve.buckley@tsi.com

Steve Buckley

Laser Ablation Molecular Isotopic Spectrometry: A New Dimension of LIBS
July 5th 2012Part of a new podcast series presented in collaboration with the Federation of Analytical Chemistry and Spectroscopy Societies (FACSS), in connection with SciX 2012 — the Great Scientific Exchange, the North American conference (39th Annual) of FACSS.
The Role of LIBS in ChemCam and SuperCam: An Interview with Kelsey Williams, Part III
May 2nd 2025In this extended Q&A interview, we sit down with Kelsey Williams, a postdoctoral researcher at Los Alamos National Laboratory (LANL), who is working on planetary instrumentation using spectroscopic techniques such as laser-induced breakdown spectroscopy (LIBS) and laser ablation molecular isotopic spectrometry (LAMIS). In Part III, Williams goes into detail about ChemCam and SuperCam and how LIBS is used in both these instruments.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)



