Multi-Quadrupole ICP-MS: Pushing Limits of Detection to the Next Decimal
For a number of elements, spectroscopic interferences can have a significant impact on the ability to achieve low detection limits in ICP-MS. We investigate the mechanisms in multi-quadrupole ICP-MS that are designed to remove these interferences.
As the limits of detection (LODs) for trace metal analysis are increasingly being pushed to the next decimal, a need exists to meet these new detection requirements without compromising accuracy or precision. Inductively coupled plasma–mass spectroscopy (ICP-MS) is often the instrument of choice for routine applications requiring ultratrace detection limits. For a number of elements, spectroscopic interferences can have a significant impact on the ability to achieve low detection limits by traditional ICP-MS systems. In this study we take a look at multi-quadrupole ICP-MS technology, with a focus on the mechanisms of removing spectral and other interferences to secure the LODs, accuracy, and precision needed for challenging applications.
Every analytical technique has its own challenges in terms of interferences and ways to remove them. This also applies to inductively coupled plasma–mass spectroscopy (ICP-MS), which is known to have a number of well-characterized spectral interferences that are generated from matrix ions, the solvents or acids being used, and the plasma gases (1). The presence of these interferences does not only have a great impact on the limits of detection (LODs), but also affects the ability to detect and accurately quantify analytes at low concentrations. In many industries, such quantification is crucial for product safety and quality control.
Spectral interferences are typically categorized into polyatomic (including oxides and hydroxides), isobaric, and doubly-charged ionic interferences. Although sample cleanup has proven to be effective at removing a number of matrix-related spectral interferences, this approach is not popular because of the prohibitive cost and time associated with it. In some cases, it is possible to simply choose an alternative isotope that does not have an interference associated with it. However, for monoisotopic elements and those at extremely low concentrations, choosing an alternative isotope is not always a viable option. Argon-based interferences are known to be effectively lowered through the use of cold or cool plasma conditions (1, 2). Unfortunately, this approach is known to suffer from matrix (non-spectral) effects, and so its use is dependent upon the application. Consequently, ICP-MS instrumentation was developed and improved over the years to effectively reduce and eliminate a number of these interferences.
The most common way to do this is through using different modes of analysis that are possible using reaction or collision cell technology. Let’s take a detailed look at how reaction and collision cellswork compared with the more traditional ways of reducing interferences.
Principles of Reaction and Collision Cells
Standard Mode
The use of correction equations was one of the first approaches used in ICP-MS analyses to correct for spectral interferences. Typically used to address isobaric and minor polyatomic interferences, the challenge with this approach is that it can result in overestimating or underestimating the interference, especially at lower analyte concentrations. Therefore, the use of correction equations does not always provide the level of accuracy needed. The name “standard mode” arose with the introduction of a cell before the analyzer quadrupole. In this mode of operation, the cell, though typically pressurized with a gas for other modes of operation, had no gas added, serving simply as an ion guide where correction equations were then applied to the resulting data.
Collision Mode
The first use of a cell pressurized with gas for ICP-MS application was a radio frequency (RF)-only driven cell, which came to be known as a collision cell. Collision cells, such as octopoles and hexapoles, are often referred to as “passive cells.” This is because these cells are not designed to have any mass separation capabilities. Instead, they were originally developed, and still continue to be used in organic mass spectrometry (tandem MS/MS), to transmit predominantly high mass ions which have resulted from the ion-fragmentation process (3). In collision mode, a non-reactive gas, such as helium, is introduced into the cell and used to pressurize it. The gas will collide with both the analyte and the polyatomic interference; however, the polyatomic ion interference typically has a larger cross-sectional diameter than the analyte ion, and will experience more collisions, lowering its kinetic energy. These polyatomic ions are then removed using a kinetic energy discrimination (KED) barrier.
This mode of operation is best used for moderate levels of interferences, and, as such, is particularly useful for the analysis of unknown matrices. The challenges associated with this mode of operation, however, are losses to analyte sensitivity and the inability to remove major interferences. For that reason, collision mode is limited to cases where the intensities of polyatomic interferences are less than four orders of magnitude higher than the analyte at that mass and where the interfering ion has a notably larger cross-sectional area than the analyte ion.
Reaction Mode
Reaction mode is typically used for applications where the interference exceeds four orders of magnitude, or where the analyte is present in extremely low concentrations, and the analysis cannot afford the signal suppression that would be introduced from a collision gas. In reaction mode, a reactive gas is introduced into the reaction cell where the choice of gas and its flow rate are selected according to reaction dynamics, which are essential for efficient interference removal. The reaction gas can either react with the interfering ion to completely remove it (exemplified in equation 1) or with the analyte ion to “mass-shift” it to a mass where there is no interference (exemplified in equation 2).
Equation 1:
An example of using a reaction gas to remove an unwanted polyatomic interference where both the interference and the analyte ions have the same mass. In this example, 35Cl16O and 51V have the same m/z of 51:
35Cl16O+ + NH3 = ClO + NH3+
51V+ + NH3= no reaction
Equation 2:
An example of using a reaction gas to mass-shift the analyte to a new mass. In this example, 40Ar35Cl and 75As both have the same m/z 75, but 75As16O+ has a higher m/z of 91:
40Ar35Cl+ + O2 = no reaction
75As+ + O2 = 75As16O+ + O
Reaction Cell with Dynamic Bandpass Mass Tuning
One of the many concerns with conventional reaction cells is that byproducts of the chemical reactions can react with the reaction gas and other ions in the cell to form new interferences. When this happens, passive cells only have limited applicability, and this is where active cells have become extremely useful. Owing to their mass filtering capabilities, quadrupole-based collision–reaction cells can be used to eject reaction by-products before they have a chance to react and form new interferences. This approach, called dynamic bandpass tuning (Universal Cell Technology), provides a clear advantage over higher order multipoles which lack the mass filtering capability to control these byproduct side reactions.
Further Reduction of Spectral Interferences
However, there are other complementary ways of reducing polyatomic and isobaric spectral interferences in ICP-MS, with the use of additional mass separation devices. Let’s take a closer look at some of these approaches.
Quadrupole Ion Deflector (QID)
Most ICP-MS instruments have a number of ion lenses that are used to filter the ion beam of unwanted photons and neutral species before they enter the cell. Historically, these lenses were designed in tandem with the interface cones. In 2010, however, the quadrupole ion deflector (QID) was developed (PerkinElmer Inc.), where an additional quadrupole was placed directly after the cones, replacing the traditional lens system. The role of this quadrupole was to deflect the ion beam 90°, while the photons and neutral species remain unaffected by the applied voltage and are removed via the vacuum. A benefit of this technology is that it behaves as an electrostatic analyzer that reduces the kinetic energy spread of the ions, and can operate with either a fixed or variable quadrupole voltage.
With a fixed voltage, a mass range of interest around the analyte ion can be transmitted to the cell, whereas ions outside this mass range are filtered off. This reduces matrix loading onto the cell or first quadrupole (depending on the instrumentation being used), and lowers the chances of new interferences forming in the cell from matrix ions. A good example of this would be for zinc (Zn), which would typically be reacted with NH3 to form zinc cluster ions (64Zn [14NH3]+3) at mass 115. By fixing the voltage of the QID to the mass window around mass 64, 114Cd+ and 115In+ ions, if present in the sample, are diverted away from the cell. As a result, a better S/N ratio for the zinc cluster ion at mass 115 is achieved.
Triple Quadrupole ICP-MS
An alternative approach to reducing interferences is to use a traditional triple quadrupole design whereby a passive cell (Q2) is placed between two quadrupole mass spectrometers. This approach, popular in organic mass spectrometry, where parent ions are subjected to collisional induced dissociation in the cell, was commercially adopted for ICP-MS in 2012. This design allows the analyst to select only the nominal mass of interest in the first analyzer quadrupole, eliminating other masses from the ion beam so that only the analyte and interfering ions remain. The interference is then removed through the use of reaction chemistry, and sometimes KED, in the collision–reaction cell (Q2), which allows the analyte to be separated from the interference in the final analyzer quadrupole. One limitation of this approach is that the cell is passive, and therefore offers no control over any potential side reactions between the reaction by-products and the gas itself or impurities in the cell gas.
Multi-Quadrupole ICP-MS
In a multi-quadrupole design, the passive cell of traditional triple quadrupole designs is replaced with an active cell possessing mass filtering capabilities. Such cells can operate in either passive (triple quadrupole) or active (multi-quadrupole) modes, and offers the ability to reject the reaction byproducts before they can potentially generate any new interferences. Figure 1 shows the layout configuration of the four quadrupoles.
Figure 1: Layout of the four quadrupoles (Q0 through Q3) in multi-quadrupole ICP-MS technology.
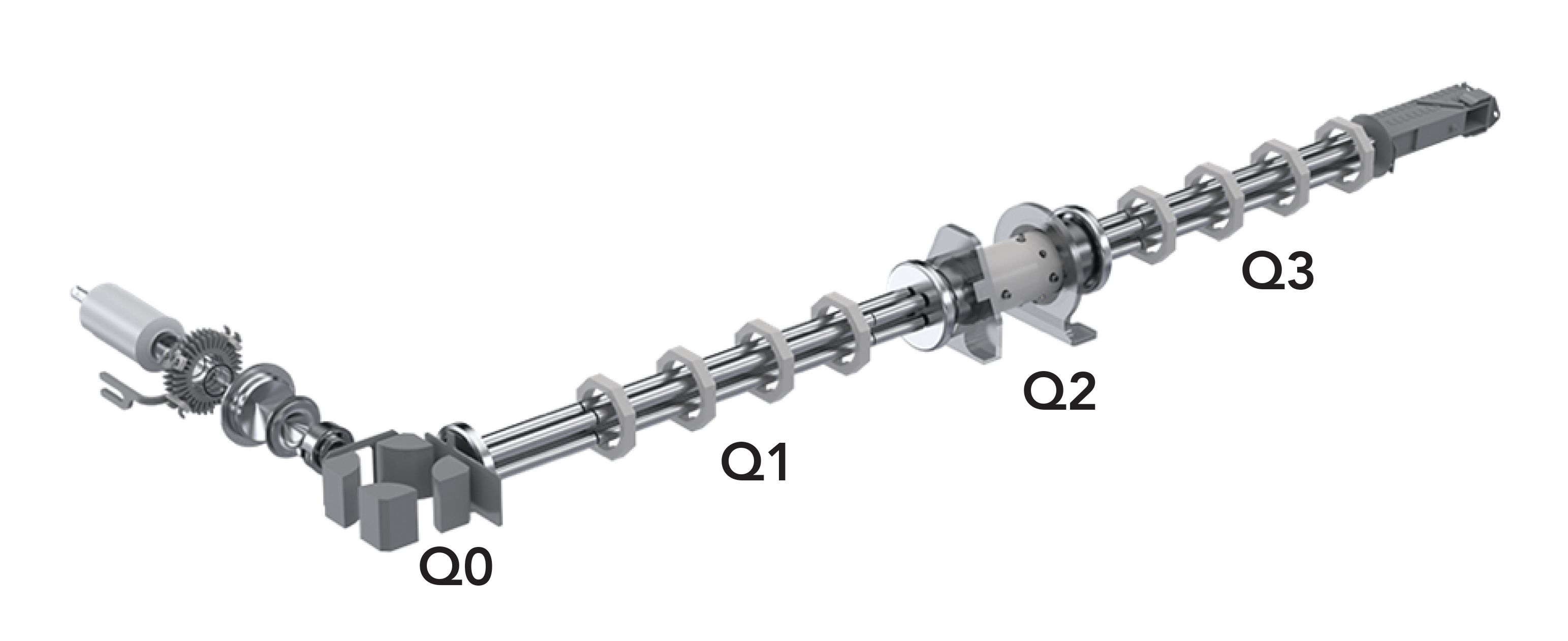
This design allows the ion beam to be cleaned up in the first quadrupole (Q0), followed by mass selection in the first transmission analyzer quadrupole (Q1),removing all interferences which are not at the desired nominal mass. After this, the analyte and any interferences that are at the same nominal mass are introduced into the active quadrupole cell (Q2) where the dynamic bandpass mass tuning capability of the cell effectively eliminates reaction byproducts before they have a chance to form new interferences. Thereafter, the interfering and analyte ions are efficiently separated in the final transmission analyzer quadrupole (Q3) in a manner that depends on the mode of operation (MS/MS or mass-shift). In this way, it not only controls the ions that enter the cell, but also controls the reactions within the cell, ensuring that byproduct ions are not formed.
Let’s explore the fundamental differences between a conventional triple quadrupole system and the multi-quadrupole technology by highlighting the determination of chromium in an organic solvent, which is particularly difficult using a passive collision–reaction cell. Note that the data in this example and all subsequent data have been generated on a NexION 5000 ICP-MS system (PerkinElmer Inc.) (4).
Chromium in an Organic Matrix
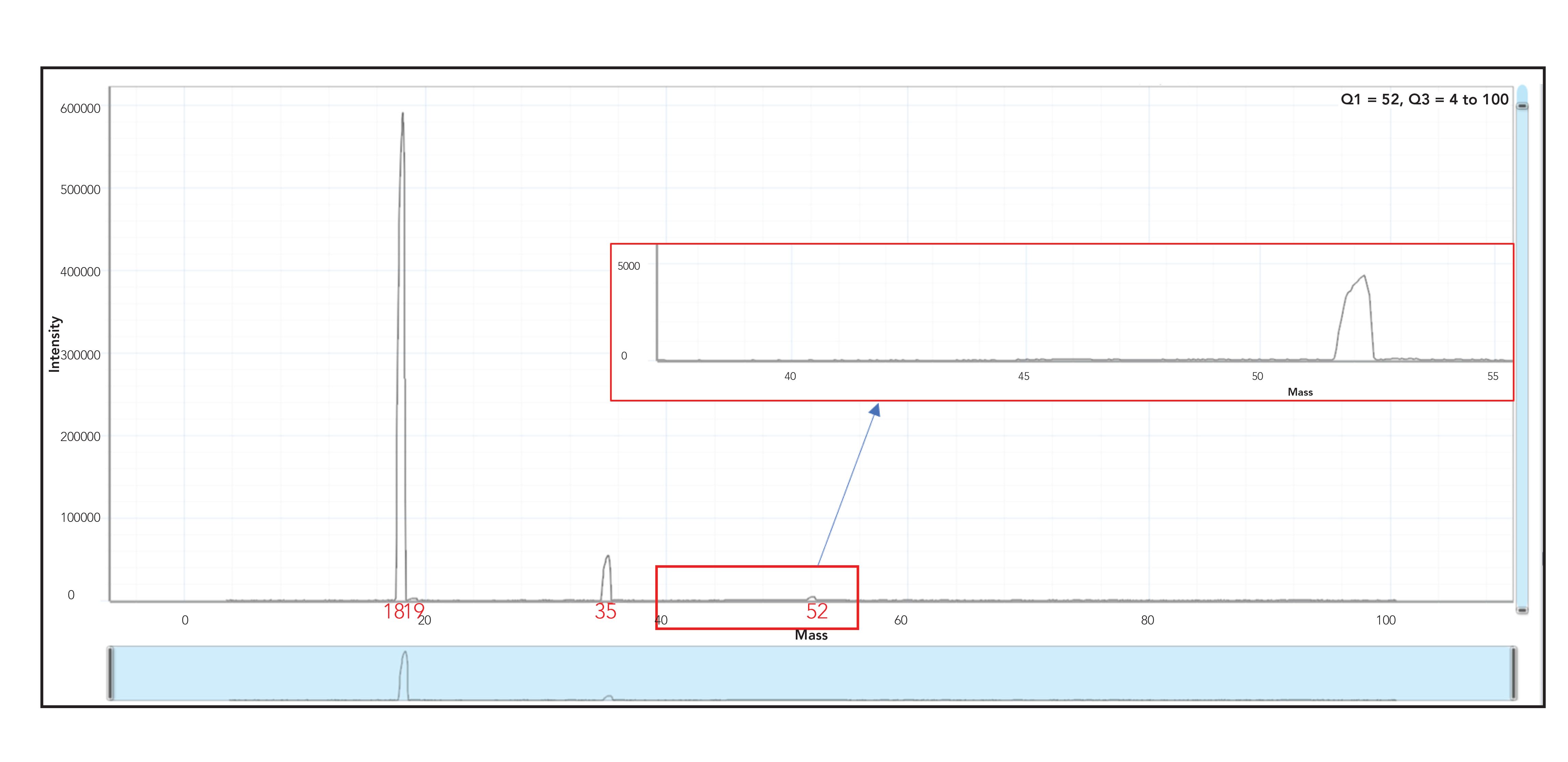
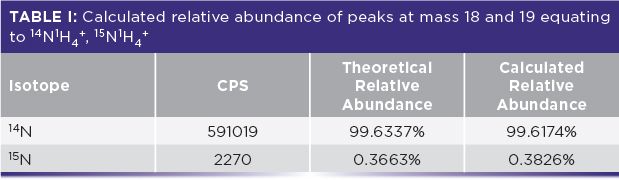
Detection limits for chromium tend to be poor in organic matrices because of the 40Ar12C polyatomic interference that forms as a result of the plasma gas and organic solvent. Figure 2 shows a product ion scan of 1% methanol, where Q1 was set to mass 52 and Q3 was set to scan the mass region from 4 to 100 amu with an NH3 gas flow of 1 mL/min. Pure ammonia was used for this reaction because it is known to produce highly efficient and reproducible reactions. In order to duplicate a passive collision cell (triple quadrupole mode), a rejection parameter q (RPq) value of 0.05 was used. Four peaks can be clearly observed at masses 18, 19, 35, and 52 (m/z 52 ca. 4500 cps) which are due to 14NH4+, 15NH4+, 14NH4+14NH3+ and 14NH4(14NH3)2+ ions respectively. The presence of these ions was confirmed by peaks at masses 18 and 19, whose abundance has the same relationship as the two isotopes of nitrogen (shown in Table I) as was determined using the formula shown in equation 3. Also contributing to the peak at mass 52 are chromium impurities in the methanol, as a result of the methanol HPLC gradient grade being used (≥ 99.8% HiPerSolv Chromanorm, VWR Chemicals BDH).
Figure 2: Product ion scan with Q1 set to mass 52, Q3 set to scan masses 4–100 in “triple quad mode” and NH3 gas flow of 1 mL/min.
Equation 3:
Calculated abundance of 15N = (15N cps)/ (14N cps + 15N cps)
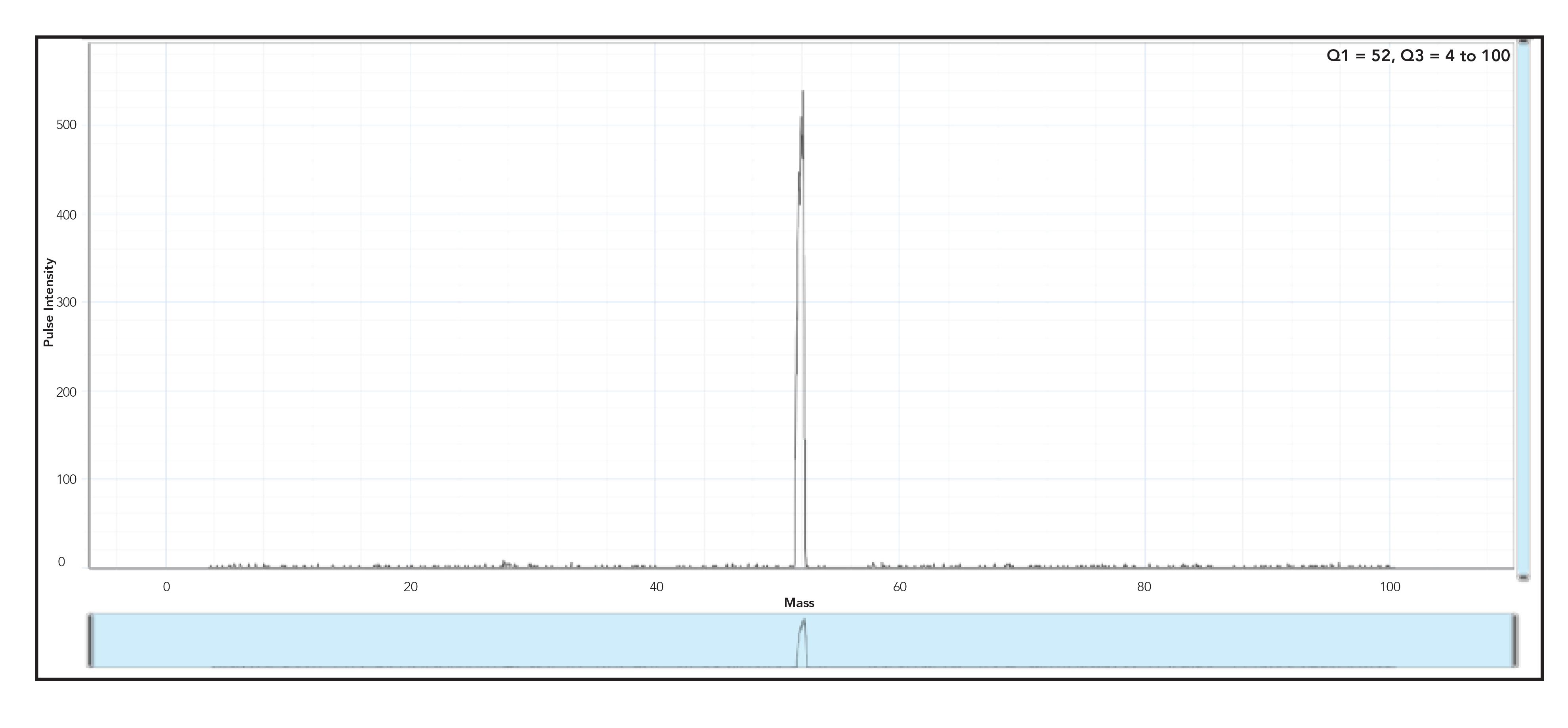
As evidenced in Figure 2, it can be clearly observed that additional species have formed from the NH3 in the cell, even though only mass 52 was allowed through Q1 and the 40Ar12C interference was removed via the reaction mode. However, once dynamic bandpass mass tuning was applied by increasing the RPq (shown in Figure 3), it can be clearly seen that all peaks that had been generated from the reaction gas are completely eliminated, leaving behind a clean and significantly smaller peak (ca. 500 cps) at mass 52, which equates to ~5 ppt Cr impurity, which could be due to the fact that methanol has been traditionally stored in stainless steel containers.
Figure 3: Product ion scan with Q1 set to mass 52, Q3 set to scan masses 4–100 with an RPq of 0.7 “multi-quad mode” and an NH3 gas flow of 1 mL/min.
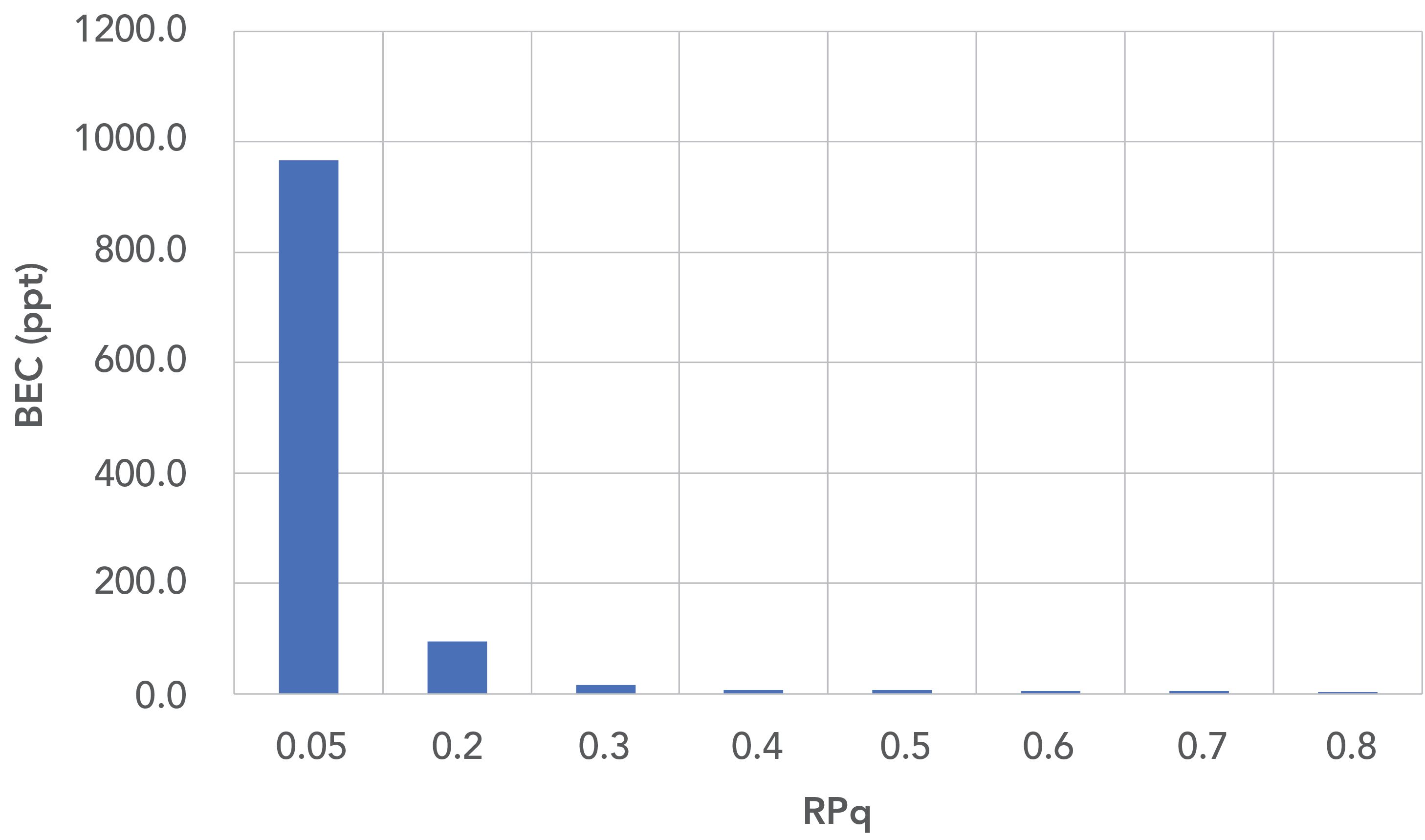
By plotting the background equivalent concentrations (BECs) against the RPq, the benefits of an active cell are apparent, as exemplified in Figure 4. Operating in “triple quad mode” at an RPq of 0.05, the BEC was around 966.7 ppt. By using the power of dynamic bandpass mass tuning a significant lowering of the BEC down to 5 ppt can be clearly observed.
Figure 4 highlights why, despite the effective way that triple quadrupole designs deal with interferences, it is challenging for these systems to reach sub-1 ppt BECs for some elements using hot plasma conditions. However, with the use of multi-quadrupole technology, BECs of <1 ppt in a hot plasma can easily be achieved.
Figure 4: Lowering of background equivalent concentrations (BECs) (ppt) for Cr with increasing RPq.
Let’s take a more detailed look at two very important application segments for ICP-MS where ultra-low detection capability and interference reduction is critically important—semiconductor and biological applications.
Semiconductor Industry
As can be expected, the cleanliness of chemicals in the semiconductor industry is critical to guaranteeing the quality and improved yields of semiconductor products (7). Ultrapure water is one such chemical and is used in a variety of different processes throughout this industry. For this reason, the Semiconductor Equipment and Materials International (SEMI F63-0918) (5) and American Society for Testing and Materials International (ASTM D5127-13) (6) have developed standards associated with the production and point of delivery of ultrapure water. The SEMI F63-0918 “Guide for Ultrapure Water Used in Semiconductor Processing,” for example, has set a target value of <1 ppt for the maximum allowable concentrations of 26 metallic contaminants with the exception of boron (50 ppt) and nickel (3 ppt).
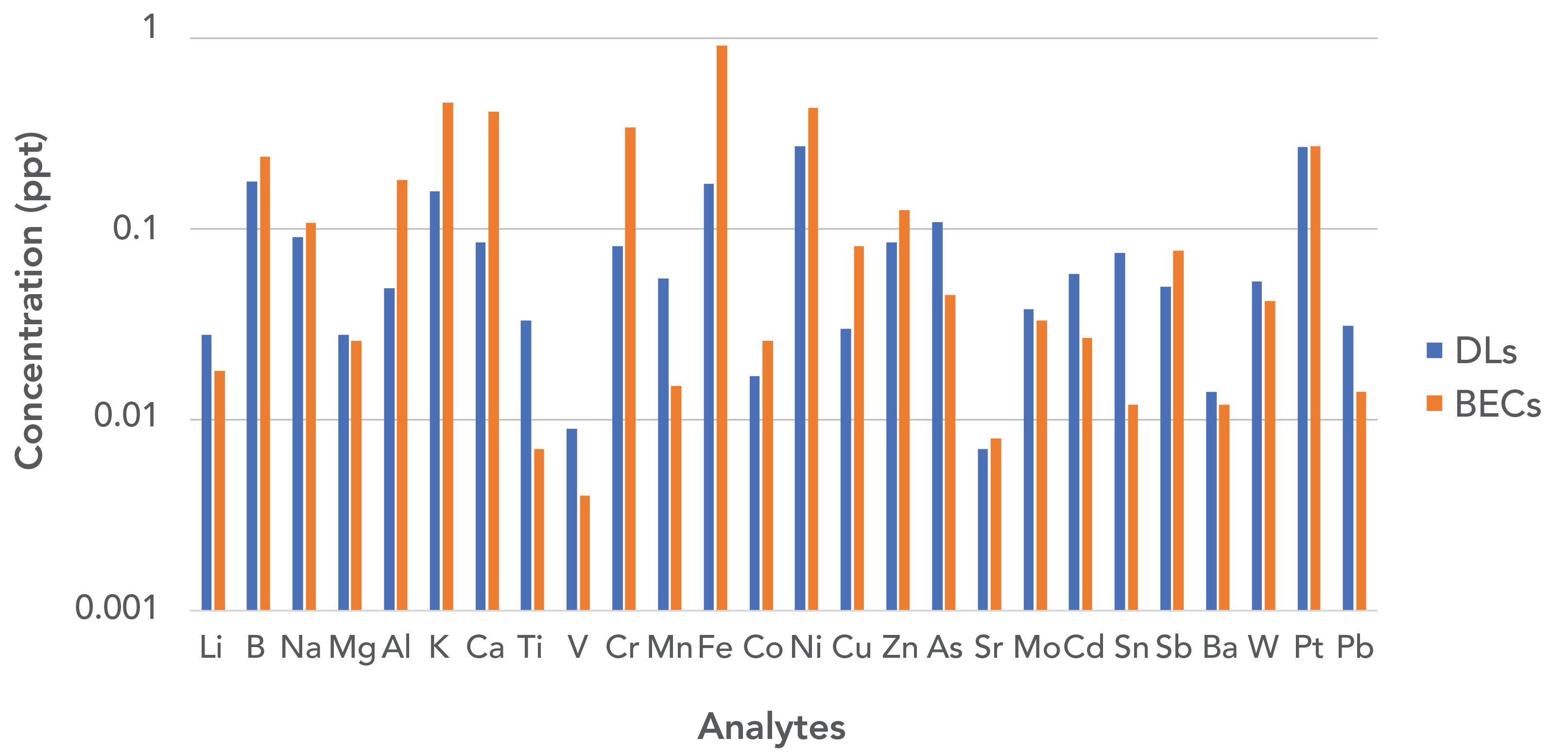
As described by Pruszkowski (7), using the multi-quadrupole ICP-MS instrument in hot plasma mode and 100% NH3 or O2 (depending upon the analyte) as reaction gases, the detection limits for all 26 elements essential to the semiconductor industry were found to be <0.3 ppt (shown in Figure 5), easily satisfying the 1 ppt requirement of SEMI and ASTM standards (7). The BECs for most of these elements were in fact found to be less than 0.5 ppt and as such demonstrates the efficiency of spectral interference removal (7).
Figure 5: Detection limits (DLs) and BECs of ultrapure water in ppt for 26 essential elements for the semiconductor industry measured in “hot plasma” mode (7).
Biological Samples
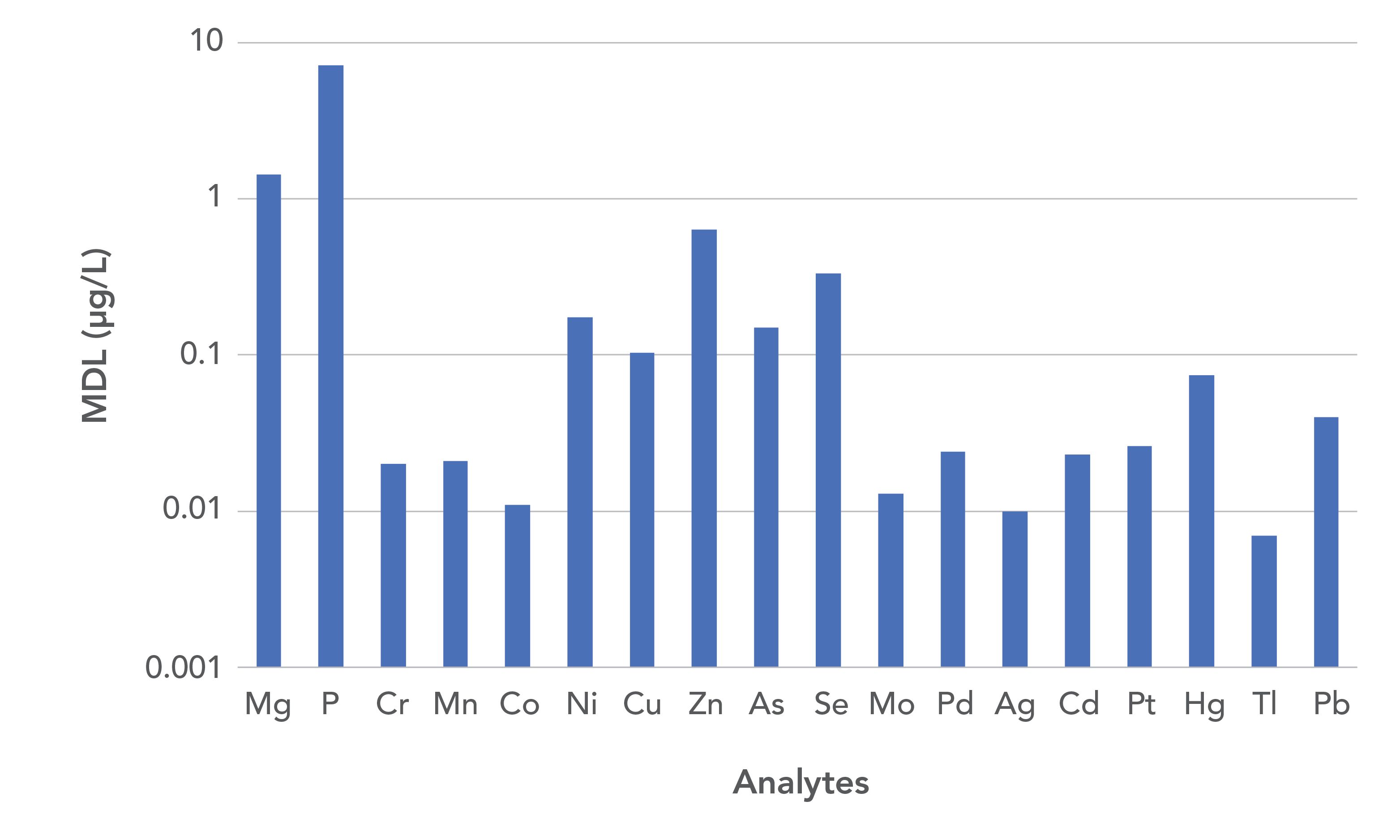
Another application area where accuracy at low levels is extremely important is in the measurement of trace metals in whole blood and serum. Blood is a complex mixture of water, red and white blood cells, proteins, hormones, glucose, and mineral salts (8). Serum is derived from blood via centrifugation, and has a similar composition despite lacking in red and white blood cells and fibrinogens (8). Aside from being a very complex mixture, blood panels are typically run to make decisions about the health of the patient where sometimes the presence of extremely low concentrations of the metal in the patient can lead to adverse effects, hence the need for good accuracy with low method detection limits (8). As described by Pruszkowski (8), Figure 6 demonstrates that, despite the complex matrix of the blood samples and diluent being used (1% HNO3 + 0.05% Triton X), extremely low MDLs were achieved (Note: MDLs were determined by analyzing the diluent 7x, and the resulting standard deviations multiplied by 50 (the dilution factor) and 3.14 (99% confidence limit)) (8).
Figure 6: Method detection limits in whole blood (8).
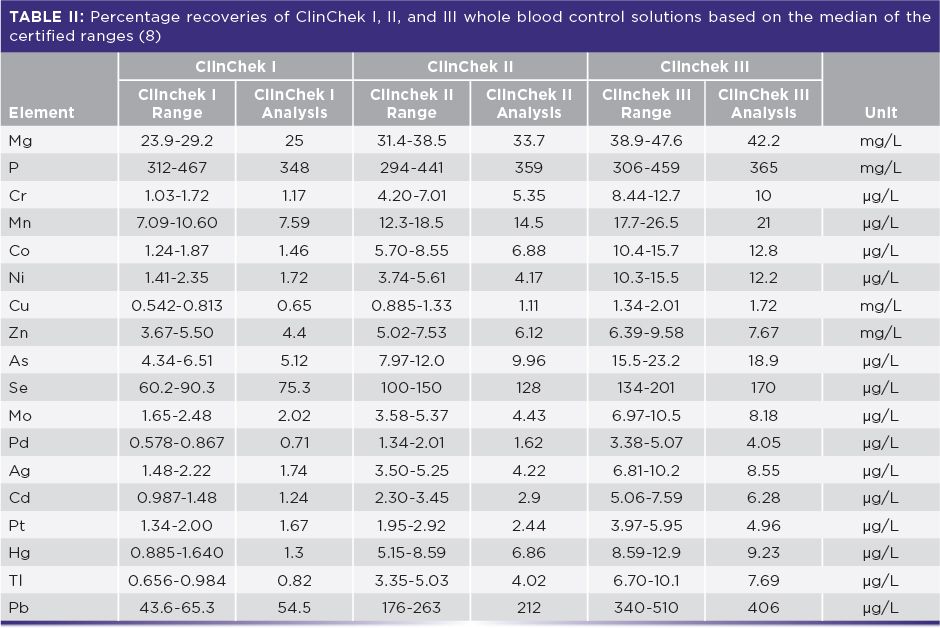
To demonstrate the accuracy of this methodology, ClinChek Levels I, II, and III whole blood control standards were analyzed using the same sample preparation procedure and comparing them against calibration curves prepared by 50x, 100x, and 200x dilutions of a ClinCal calibration standard (8). The results in Table II show percent recoveries compared to the normalized results of the mean certificate values. It can be seen that recoveries are well within the certified values (8).
Conclusion
In conclusion, multi-quadrupole ICP-MS technology offers a distinct advantage over traditional approaches in the removal of spectral interferences, by not only controlling the ions that enter the cell, but also the reaction products within the cell. For example, there is an improvement of approximately two orders of magnitude in the background equivalent concentrations when the bandpass is applied for chromium, which has a direct impact on the detection limit. This allows for the accurate sub-ppt quantification of analytes in the semiconductor industry even in the hot plasma mode, which does not suffer from the same non-spectral matrix effects as are often observed when using cold plasma conditions. Furthermore, this technology can be extremely useful in the field of biomonitoring studies, effectively removing the spectral interferences to provide high accuracy at low levels in complex sample matrices. The application of this technology is not limited to these application fields, but can be extended to other applications where spectral interferences are a significant limitation such as environmental, geochemical, petrochemical, and academic research.
References
- R. Thomas, Practical Guide to ICP-MS (Marcel Dekker Inc., New York, New York, 2004).
- K. Sakata and K. Kawabata, Spectrochim. Acta49B, 1027 (1994).
- S.D. Tanner and V.I. Baranov, J. Am. Soc. Mass Spectrom. 10, 1083–1094 (1999).
- PerkinElmer. NexION 5000 ICP-MS Product Technical Note. https://www.perkinelmer.com/Product/nexion-5000-icp-ms-n8160010 (accessed August 2020).
- ASTM D5127-13. Standard Guide for Ultra-Pure Water Used in the Electronics and Semiconductor Industries. https://www.astm.org/Standards/D5127.htm (accessed August 2020).
- SEMI F63-0918. Guide for ultrapure water used in semiconductor processing. https://store-us.semi.org/products/f06300-semi-f63-guide-for-ultrapure-water-used-in-semiconductor-processing (accessed August 2020).
- E. Pruszkowski, PerkinElmer Application Note: Characterization of Ultrapure Water using NexION 5000 ICP-MS. https://www.perkinelmer.com/lab-solutions/resources/docs/APP_Nexion5000-ICP-MS-SemiconUltrapureWater.pdf (accessed August 2020). 2020A.
- E.Pruszkowski, PerkinElmer Application Note: Analysis of Blood using NexION 5000 ICP-MS. https://www.perkinelmer.com/lab-solutions/resources/docs/app_nexion5000-icp-ms-biomonitoringblood.pdf (accessed August 2020). 2020B., Inc.

Eve Kroukamp is an Associate Product Leader for ICP-MS at PerkinElmer, Inc.

Fadi Abou Shakra is the Portfolio Director for ICP-MS at PerkinElmer, Inc.

Robert Thomas is the principal of Scientific Solutions, an educational consulting company that serves the training and writing needs of the trace element user community. He has worked in the field of atomic and mass spectroscopy for more than 45 years, including 24 years for a manufacturer of atomic spectroscopic instrumentation. Rob has written more than 100 scientific publications, including a 15-part tutorial series, A Beginners Guide to ICP-MS. He is also the editor and frequent contributor of the Atomic Perspectives column in Spectroscopy magazine. In addition, Rob has authored three textbooks on ICP-MS and in 2018 completed his fourth book, entitled Measuring Elemental Impurities in Pharmaceuticals. He has spent the past two years researching and writing a new book, Measuring Heavy Metal Contaminants in Cannabis and Hemp, which was published in September 2020. Rob has an advanced degree in analytical chemistry from the University of Wales, UK, and is also a Fellow of the Royal Society of Chemistry (FRSC) and a Chartered Chemist (CChem).

Atomic Perspectives: Highlights from Recent Columns
March 3rd 2025“Atomic Perspectives,” provides tutorials and updates on new analytical atomic spectroscopy techniques in a broad range of applications, including environmental analysis, food and beverage analysis, and space exploration, to name a few. Here, we present a compilation of some of the most popular columns.
Pittcon 2025: Highlighting Talks on Atomic Spectroscopy
February 26th 2025At Pittcon this year, there will be numerous sessions dedicated to spotlighting the latest research that uses atomic spectroscopy or elemental analysis techniques. We highlight some of these talks below that might pique the interest of spectroscopists and researchers attending the conference this year.