Tandem LA–LIBS Coupled to ICP-MS for Comprehensive Analysis of Tumor Samples
Application Notebook
Elemental imaging is regarded as a valuable approach for a wide application range in modern medicine. Using tandem laser-ablation laser-induced breakdown spectroscopy (LA–LIBS) coupled to inductively coupled plasma–mass spectrometry (ICP-MS), high-sensitivity detection of trace metals can be combined with the possibility of analyzing nonmetallic bulk components of biological samples (for example, carbon, hydrogen, and oxygen). In this work, the applicability of the tandem LA–LIBS approach for the laterally resolved elemental analysis of a mouse model tumor sample after administering cytostatic medication is demonstrated. Results show that trace elements can be detected using the LA-ICP-MS domain of the setup while major components of the samples are analyzed simultaneously using LIBS. By expanding the analyte range covered during one analysis, information could be extracted from the data set that is not accessible to either of the stand-alone analysis methods.
Laser-ablation inductively coupled plasma–mass spectrometry (LA-ICP-MS) is accepted nowadays as one of the prime methods for trace-element imaging in biological samples (1,2). The method's excellent limits of detection below the microgram-per-gram range for most elements paired with a wide dynamic range and a number of valid approaches for quantification make this method the perfect choice when trace-element distributions need to be assessed (3). Thereby, endogenous elements (4,5) as well as analytes originating from external sources (for example, drug administration) have already been analyzed (6,7). During the past few years, more-sophisticated quantification strategies have successfully been developed to allow simpler and more accurate analysis (8,9). Achievable lateral resolutions are descending toward the low micrometer range and below (10,11), driven by the development of ultrafast washout cells (12,13). However, one weakness of LA-ICP-MS remains: the possibility to analyze nonmetals, especially those present in biological samples. Although the analysis of phosphorus is rather unproblematic, investigations of sulfur and carbon are already more complicated: They are severely influenced by spectral interferences (for example, 32S+ – 16O16O+, 34S+ – 16O18O+). In particular for carbon, the high first ionization potential is also a problem. When aiming to detect hydrogen, oxygen, or nitrogen using LA-ICP-MS, the analysis will not be successful at all because of the high background signals and weak ionization of these analytes in the Ar plasma. Thus, alternative methods are necessary if analysis of the biological bulk elements is desired.
In contrast to LA-ICP-MS, laser-induced breakdown spectroscopy (LIBS) is a technique far less commonly used for imaging studies on biological tissues (14,15). Although the limits of detection for metallic analytes are usually higher compared to LA-ICP-MS, the method provides the possibility to detect biological bulk elements such as carbon, hydrogen, and oxygen with sufficient sensitivity. A combination of both techniques is realized in so-called tandem LA–LIBS systems, which, when coupled to ICP-MS for analyte detection, extract the benefits of both the stand-alone methods described above (16,17). When irradiating the sample surface with a short-pulse laser beam, the light emitted by the laser-induced plasma is collected and analyzed by optical means-the LIBS domain of the system. In parallel, the generated sample aerosol is transferred to an ICP-MS system, like in regular LA-ICP-MS analysis. Such multimodal chemical imaging approaches, where two or more methods are used to analyze the same sample, have already been shown to deliver insightful results for biological questions. For example, the combination of LA-ICP-MS and organic mass spectrometry (such as matrix-assisted laser desorption–ionization mass spectrometry [MALDI-MS]) can deliver molecular as well as elemental distribution information from one specimen (18).
In this study, tandem LA–LIBS coupled to ICP-MS has been used for multimodal elemental mapping of a tumor sample obtained from a mouse model. The specimen underwent a combination treatment with two cytostatic drugs, sunitinib and cisplatin, with the aim to increase drug efficacy. The use of such a hybrid analysis approach could provide a complete elemental analysis of tissue samples including trace and minor elements as well as bulk elements in biological samples. Because of the excellent sensitivity of LA-ICP-MS, platinum originating from the administered drug could also be detected in the tissue. Within this study, it could be shown that gaining information about the bulk element distributions can essentially increase the value of elemental imaging studies.
Experimental
Animal Experiments and Sample Preparation
The C26 mouse colon adenocarcinoma cell line (Cell Line Service, Mason Research Institute) was cultured in Roswell Park Memorial Institute (RPMI) 1640 medium with 10% fetal bovine serum and 1% penicillin–streptomycin (all from Sigma Aldrich) in a humidified atmosphere at 37 °C, 5% CO2. Groups of six 8-week-old female Balb/C mice from the colony of the National Oncology Institute in Budapest were inoculated subcutaneously with 2 × 106 C26 cells.
All animal-model protocols were developed and conducted in accordance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines and the animal welfare regulations of the Department of Tumor Biology, National Koranyi Institute of Pulmonology (permission number: 22.1/1268/3/2010). Mice were kept on a daily 12-h light/12-h dark cycle and held in a conventional animal house in microisolator cages with water and laboratory chow ad libitum.
Sunitinib was purchased from LC Laboratories (CAS. No. 557795-19-4) at >99% purity and suspended in 2% carboxymethylcellulose with 2-mg/mL methyl-4 hydroxibenzoate (both from Sigma Aldrich). Cisplatin was purchased from Accord Healthcare (Ma No.: OGYI-T-21728/01).
Sunitinib treatment began seven days after tumor cell injection and was performed orally with a feeding tube once daily at a dose of 80 mg/kg each day for two weeks. With the final dosage of sunitinib, mice also received a bolus of cisplatin intraperitoneally at a dose of 10 mg/kg. The animals were sacrificed 3 h after the last treatment. Tumors were removed and snap frozen by submerging the tissues into dry ice–cooled isopentane. Frozen tissues were stored at -80 °C until utilization.
Cryo-cut sections of tumor tissue with a thickness of 10 µm were deposited onto silicon wafers. Silicon substrates were surface modified with (3-aminopropyl)triethoxysilane (APES) to optimize adhesion of the tissue section according to a protocol used in a previous study (19). Before analysis, the samples were coated with a thin gold layer to be used as a pseudo-internal standard during LA-ICP-MS data acquisition. For coating, an Agar B7340 sputter coater (Agar Scientific Limited) equipped with a gold sputter target was used. Samples were sputtered for 10 s (timer controlled) at a pressure of 0.1 mbar with a constant distance of 3.0 cm between sputter target and sample. This procedure has already been reported earlier to be suitable for signal normalization in LA-ICP-MS imaging (20).
Sample Analysis
Tandem LA–LIBS experiments were performed using a commercially available J200 tandem LA–LIBS system (Applied Spectra, Inc.) equipped with a frequency quadrupled Nd:YAG laser operating at a wavelength of 266 nm. Element-specific radiation emitted from the laser-induced plasma was collected by optical fibers and analyzed using a Czerny-Turner–type spectrometer with six-channel charge-coupled device (CCD) detection. This setup enabled the analysis of the full spectral range between 190 and 1050 nm (spectral resolution approximately 0.1 nm) for every laser shot. Axiom software provided by the manufacturer of the instrument was used for collection of the LIBS data. The system was connected to an iCAP Qc ICP-MS system (Thermo Fisher Scientific) using polytetrafluoroethylene (PTFE) tubing with a 3-mm inner diameter and a length of 1.5 m. A helium flow rate of 0.9 L/min provided optimal washout properties for the sample aerosol, and 0.3 L/min argon was admixed to the helium flow directly after the ablation chamber as make-up gas. ICP-MS data were acquired in time-resolved mode using Qtegra software provided by the manufacturer of the system. Instrumental parameters of the LIBS system were optimized in preliminary experiments: Cryo-cuts of homogenized tissue samples were ablated and the parameters (that is, laser output energy and spectrometer gate delay) were selected to detect the maximum background corrected signal for carbon at the analytical wavelength of 247.856 nm, because the carbon signal was the weakest of all analytes aimed to be analyzed by LIBS. Before every analysis, instrumental parameters of the ICP-MS system were optimized by ablating the National Institute of Standards and Technology (NIST) 612 certified reference material (trace elements in glass) with the optimized laser settings for the LIBS domain. Settings were tuned to achieve the maximum signal intensity for 23Na, 56Fe, and 63Cu; typical instrumental parameters are shown in Table I.
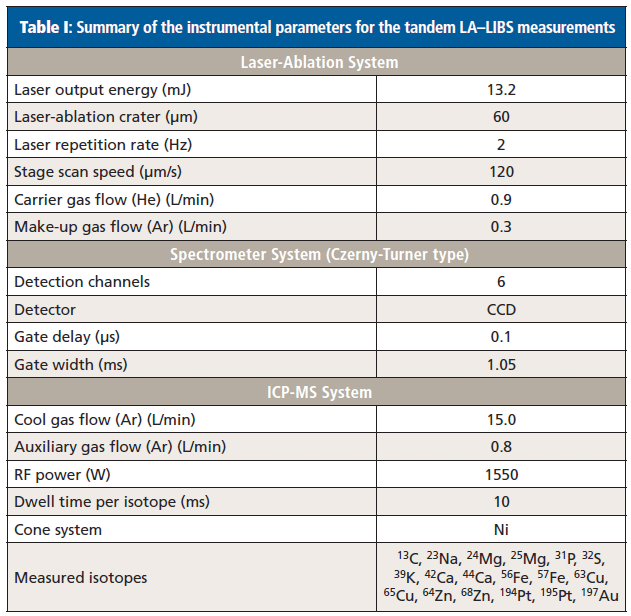
Table I: Summary of the instrumental parameters for the tandem LA–LIBS measurements
Samples were ablated using parallel line scans without spacing between consecutive lines; all lines were scanned in the same direction. Stage scan speed was adjusted to traverse the complete diameter of the laser beam between two adjacent laser shots. The complete sample was ablated in one run of analysis. Settings were adjusted to obtain a lateral resolution of 60 µm per pixel for the final elemental distribution images obtained by both LIBS and LA-ICP-MS.
Image Construction and Coregistration
The acquired time-dependent intensity plots by LA-ICP-MS were transformed into elemental distribution images using ImageLab software (v1.98, Epina GmbH) and raw data were normalized by the recorded gold signal. The LIBS dataset, where each spectrum corresponds to one discrete position on the sample, was analyzed using Aurora software (v.18, Applied Spectra, Inc.). After integration and background correction of selected analyte signals, two-dimensional (2D) maps were exported to ImageLab for further data processing. Using the known spatial coordinates of features and the measured sample area, the distribution images from the two analysis modes could be coregistered along with a microscopic image of the sample taken before ablation.
Results
Elemental Mapping of an Adenocarcinoma Tumor Sample
An approximately 5 mm × 5 mm large mouse model adenocarcinoma tumor sample (10 µm thickness) treated with the anticancer drugs cisplatin and sunitinib was investigated in this study. A 60-µm lateral resolution of the elemental distribution images was sufficient for reliable interpretation of the results and keeping analysis time as low as possible. Analysis of the shown sample required around 1.5 h, which is also acceptable for measurements of a larger sample pool.
Using the LIBS domain of the setup, analysis of the biological bulk elements carbon, hydrogen, and oxygen was feasible. Additionally, alkaline and earth alkaline metals usually occurring at elevated concentrations in biological systems (that is, Na, K, Mg, and Ca) could be well detected using this technique. Signals of the strongest emission lines of those elements were well above background level on all areas of the sample with a signal-to-noise ratio always above 5.0. The silicon sample carrier exhibited no significant background signals for the investigated emission lines. Average background corrected signal intensities ranged from 2000 cts for C to 50,000 cts for Na. Elements occurring at lower concentrations were analyzed using the LA-ICP-MS modality of the setup. Namely, those were phosphorus and sulfur alongside with endogenous trace metals (such as Fe, Cu, Zn) and platinum originating from cisplatin treatment of the mouse. Also for these elements, the silicon carriers did not exhibit significant background signals, which allowed sensitive detection. With average signal intensities ranging between 5000 cts for Pt and 200,000 cts for P, all elements of interest could be clearly detected above background level. A compilation of the elements detected by the two modalities is shown in Table II.
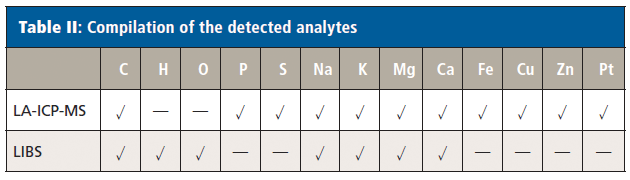
Table II: Compilation of the detected analytes
Using the earlier described procedure, elemental distribution images from the two modalities were combined. Selected elemental maps are shown in Figure 1. While the bulk elements carbon (Figure 1b) and hydrogen (Figure 1c) show rather homogenous distribution, oxygen (Figure 1d) and phosphorus (Figure 1f) are not evenly distributed across the sample. A reduced intensity can be seen in the bottom area of the sample. Magnesium (Figure 1e) and zinc (Figure 1h) follow a similar pattern. Sodium, as well as potassium (not shown) are again rather evenly distributed across the tissue sample. Compared to a tendency of depletion in the distinct bottom part of the sample for some elements, the platinum signal is elevated in this area.
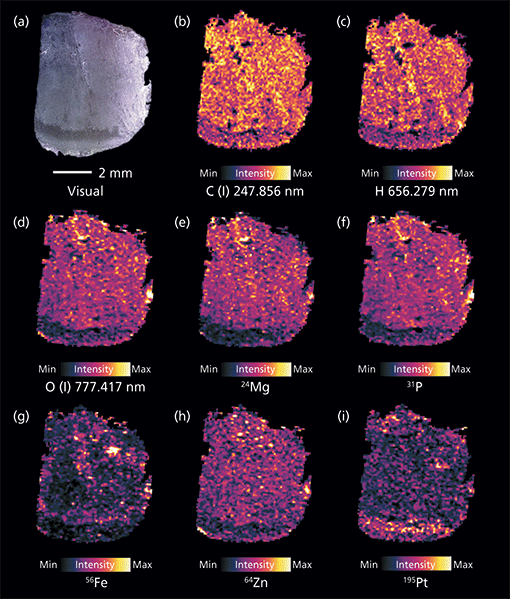
Figure 1: Compilation of selected elemental distributions measured on an adenocarcinoma sample using tandem LA-LIBS coupled to ICP-MS. Shown are (a) a microscopic image of the sample alongside the distributions of (b) carbon, (c) hydrogen, and (d) oxygen measured by LIBS (background corrected signal intensities), and (e) magnesium, (f) phosphorus, (g) iron, (h) zinc, and (i) platinum detected by LA-ICP-MS (gold-normalized signal intensities).
Discussion
Comparison of Elemental Maps Derived From LIBS and LA-ICP-MS Measurements
Some elements (see Table II) could be detected using both modalities of the measurement system. Optimally, the two obtained elemental distributions should deliver the same result-a requirement for reliable results. Localization of corresponding pixels as well as relative signal intensities should correlate well. Besides a visual comparison (Figures 2a and 2b), linear correlation of corresponding pixels is also a simple tool for determining similarity and spatial matching of two images (Figure 2c).
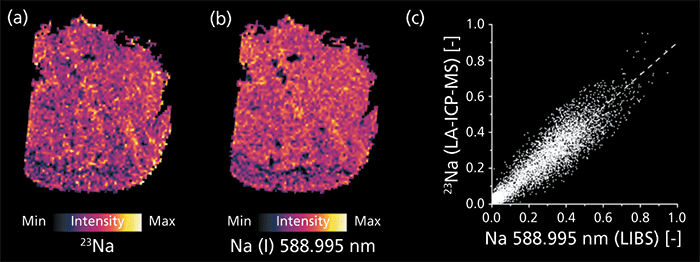
Figure 2: Distributions of sodium measured by (a) LA-ICP-MS and (b) LIBS. Signal intensities are normalized to 1 with regards to the highest signal detected over the complete sample; good correlation of the two modalities is demonstrated by (c) a pixel-by-pixel regression.
As an example, the detected Na distributions are examined here. When visually comparing the elemental distributions measured simultaneously by LIBS and LA-ICP-MS, a good correlation is already expected. The shape of the tissue sample as well as the fine structures represented by varying relative signal intensities match well. This finding is underlined by the determination of the image similarity using linear correlation. The high numerical value of the Pearson's correlation coefficient (R = 0.918) and normally distributed residuals from the linear fitting function (Kolmogorov-Smirnov test, α = 0.99) do not indicate a significant difference between the two distribution images, neither from their positioning nor their relative signal intensities. Comparable findings can be reported for the other elements detected using both modalities. The high similarity of all simultaneously detected analytes demonstrates the viability of this multimodal elemental imaging approach. The combined data set thus offers valid information about the measured analyte distributions.
Apoptosis in Adenocarcinoma Samples
The most significant area in the microscopic image, as well as the elemental distribution images, is the bottom area of the tissue sample (Figure 1). Histological stainings of consecutive thin-cuts show that the area in the microscopic image that appears slightly darker than the rest of the sample represents an apoptotic part of the tumor (Figure 1a). Here, signal intensities of phosphorus, oxygen, and also metals are significantly reduced in comparison to the remaining tissue section. This result is interesting because it shows a good correlation of the phosphorus (LA-ICP-MS) and oxygen distributions (LIBS). This finding points toward the fact that the abundance of phosphate is reduced in the apoptotic area compared to the rest of the sample. Such a finding is well explained by the processes occurring during apoptosis, which is a stage of a cell's life cycle also known as programmed cell death (21). At the beginning of this phase, cellular functions are down-regulated leading to decreased metabolic activity, which is linked to the presence of phosphates in different forms: Adenosintriphosphate (ATP) and its derivatives, as well as desoxyribonucleic acid (DNA) and ribonucleic acid (RNA) are just a few examples. Thus, lower phosphate content in apoptotic areas can be expected. Similar findings can be reported for physiologically relevant (trace-level) metals, such as magnesium or zinc, which also play a major role for proper enzyme functioning (22,23). Apoptosis is most likely induced by the treatment using sunitinib, which should inhibit cellular signaling by targeting multiple receptor tyrosine kinases (RTKs). Conversely, elevated concentrations of platinum, originating from the cisplatin treatment, can be found in the apoptotic tissue area. Possible resistance mechanisms that can lead to drug removal from the tumor sites are impaired in the apoptotic state, leading to a more efficient impact of the drug in such combinational treatment. Compared to the above described elements, bulk elements like carbon and hydrogen show rather uniform distribution, indicating similar composition and density of the tissue across the complete investigated sample.
Conclusion
In the past, trace-metal mapping in biological tissues has already been shown to provide valuable results for further medical interpretation. Deeper knowledge of the function of metals in tissues is believed to be able to provide valuable insight into complex biological processes, such as tumor genesis and drug efficacy. With additional information on biological bulk elements, the value of elemental imaging in the life sciences can be further expanded, as shown in the present example for tumor imaging. Not only can the information depth of LA-ICP-MS imaging be expanded by adding the LIBS domain, but because of the simultaneous measurement of a full spectrum with every laser shot, the analytes do not have to be selected before analysis as is usual with quadrupole ICP-MS instrumentation. This advantage gives the possibility of mapping all elements that can be detected without jeopardizing the quality of the measured results. Additionally, the number of elements monitored using LA-ICP-MS can be reduced, leading to shorter cycling times and a longer data acquisition time for low-concentration elements. In further studies, tandem LA–LIBS coupled to ICP-MS can be used for quantitative imaging applications and for answering deeper medical questions.
The findings described above illustrate the excellent suitability of tandem LA–LIBS imaging for biological applications. Using the exceptional features of such a multimodal approach it was possible to map both bulk and trace elements in one run of analysis. For instance, the good correlation between the phosphorus distribution measured by LA-ICP-MS and the oxygen distribution acquired by LIBS, along with a suitable biological interpretation for this finding highlights the value of multimodal analysis approaches. This example outlines the fact that the simultaneous use of LA-ICP-MS and LIBS can be beneficial-especially when the stand-alone techniques cannot detect all the elements of interest.
References
(1) J.S. Becker, Methods Mol. Biol. 656, 51–82 (2010).
(2) I. Konz, B. Fernandez, M.L. Fernandez, R. Pereiro, and A. Sanz-Medel, Anal. Bioanal. Chem. 403(8), 2113–2125 (2012).
(3) R. Russo, Talanta 57(3), 425–451 (2002).
(4) J. Lear, D.J. Hare, F. Fryer, P.A. Adlard, D.I. Finkelstein, and P.A. Doble, Anal. Chem. 84(15), 6707–6714 (2012).
(5) J.S. Becker et al., Anal. Chem. 82(22), 9528–9533 (2010).
(6) M. Bonta, H. Lohninger, V. Laszlo, B. Hegedus, and A. Limbeck, J. Anal. At. Spectrom. 29(11), 2159–2167 (2014).
(7) S. Theiner et al., Metallomics: Integrated Biometal Science 7(8), 1256–1264 (2015).
(8) D. Hare, C. Austin, and P. Doble, The Analyst 137(7), 1527–1537 (2012).
(9) A. Limbeck, P. Galler, M. Bonta, G. Bauer, W. Nischkauer, and F. Vanhaecke, Anal. Bioanal. Chem. 407(22), 6593–6617 (2015).
(10) C. Giesen et al., Nat. Meth. 11(4), 417–422 (2014).
(11) S.J. Van Malderen, E. Vergucht, M. De Rijcke, C. Janssen, L. Vincze, and F. Vanhaecke, Anal. Chem. 88(11), 5783–5789 (2016).
(12) H.A.O. Wang et al., Anal. Chem. 85(21), 10107–10116 (2013).
(13) S.J.M. Van Malderen, J.T. Van Elteren, and F. Vanhaecke, J. Anal. At. Spectrom. 30(1), 119–125 (2015).
(14) V. Motto-Ros et al., Spectrochim. Acta, Part B 87, 168–174 (2013).
(15) L. Sancey et al., Sci. Rep. 4, article number: 6065 (2014).
(16) M. Dong et al., Spectrochim. Acta, Part B 109, 44–50 (2015).
(17) K. Subedi, T. Trejos, and J. Almirall, Spectrochim. Acta, Part B 103–104, 76–83 (2015).
(18) J. Bianga et al., Metallomics: Integrated Biometal Science 6(8), 1382–1386 (2014).
(19) M. Bonta, J.J. Gonzalez, C. Derrick Quarles, R.E. Russo, B. Hegedus, and A. Limbeck, J. Anal. At. Spectrom . 31(1), 252–258 (2016).
(20) M. Bonta, H. Lohninger, M. Marchetti-Deschmann, and A. Limbeck, The Analyst 139(6), 1521–1531 (2014).
(21) D.J. Fawthrop, A.R. Boobis, and D.S. Davies, Arch. Toxicol. 65(6), 437–444 (1991).
(22) J. Durlach, Magnesium in Clinical Practice (J. Libbey, London, UK, 1988).
(23) J.E. Coleman, Annu. Rev. Biochem. 61, 897–946 (1992).
Maximilian Bonta and Andreas Limbeck are with TU Wien, Institute of Chemical Technologies and Analytics in Vienna, Austria. Szilvia Török is with the Department of Tumor Biology at the National Koranyi Institute of Pulmonology in Budapest, Hungary. Balazs Döme is with the Department of Tumor Biology at the National Koranyi Institute of Pulmonology, the Division of Thoracic Surgery, Department of Surgery, Comprehensive Cancer Center at the Medical University of Vienna in Austria, the Department of Biomedical Imaging and Image-guided Therapy, Division of Molecular and Gender Imaging, Medical University of Vienna, and the Department of Thoracic Surgery, Semmelweis University and National Institute of Oncology in Budapest, Hungary. Direct correspondence to: andreas.limbeck@tuwien.ac.at

New Study Reveals Insights into Phenol’s Behavior in Ice
April 16th 2025A new study published in Spectrochimica Acta Part A by Dominik Heger and colleagues at Masaryk University reveals that phenol's photophysical properties change significantly when frozen, potentially enabling its breakdown by sunlight in icy environments.