Temperature Induced Changes in Clays Measured by Diamond ATR FT-IR Spectroscopy
Application Notebook
Increasing temperature can cause chemical changes, alter reaction rates, concentrate an aqueous sample, or evaporate a solvent. These transformations can be readily monitored by diamond ATR spectroscopy. This note explores the use of a diamond ATR to examine heat-induced changes in the clay.
Increasing temperature can cause chemical changes, alter reaction rates, concentrate an aqueous sample, or evaporate a solvent. These transformations can be readily monitored by diamond ATR spectroscopy. This note explores the use of a diamond ATR to examine heat-induced changes in the clay.
Experimental
The measurements were carried out using a diamond single reflection ATR, the MVP-Pro™ with its heated diamond crystal holder and temperature controller (Figure 1) installed in a commercial FT-IR spectrometer equipped with a DTGS detector. The temperature controller was autotuned for operation to 200 °C. Then the desired set point was selected as needed. Spectra were collected at an 8 cm-1 resolution, a gain of 8, and signal averaged over 32 scans. A small mound of the montmorillonite ("otay"; SCa-3, Clay Minerals Society) was pressed against the diamond ATR using the maximum force delivered by the built-in pressure applicator.

Figure 1: MVP-Pro Star shown with heated cell and temperature controller.
Results and Discussion
Figure 2 shows the spectra of otay before and after heating. Clays such as otay are hygroscopic and easily absorb water at room temperature. The resulting water peak in the 3000 cm-1 to 3400 cm-1 region can obscure the characteristic C–H stretch. Heating the sample to 200 °C for 5 min drives off the water, making the C–H peaks in these samples emerge or become more distinct. In all of the figures, blue signifies room temperature spectra while red denotes heated spectra. The portion of the spectrum in the 2500 cm-1 to 2000 cm-1 region is not shown due to the absorbance of diamond in that region.
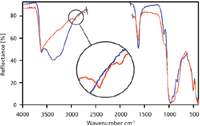
Figure 2: Otay spectra at 26 °C (blue) and 200 °C (red).
Conclusion
Heated diamond ATR accessories can be used effectively to examine evaporation upon heating. Diamond ATR accessories such as the MVP-Pro™ are powerful tools for examining heat-induced chemical transformations.
Harrik Scientific Products, Inc.
141 Tompkins Ave., P.O. Box 277, Pleasantville, NY 10570
tel. (800) 248-3847
Website: www.harricksci.com

Thermo Fisher Scientists Highlight the Latest Advances in Process Monitoring with Raman Spectroscopy
April 1st 2025In this exclusive Spectroscopy interview, John Richmond and Tom Dearing of Thermo Fisher Scientific discuss the company’s Raman technology and the latest trends for process monitoring across various applications.
A Seamless Trace Elemental Analysis Prescription for Quality Pharmaceuticals
March 31st 2025Quality assurance and quality control (QA/QC) are essential in pharmaceutical manufacturing to ensure compliance with standards like United States Pharmacopoeia <232> and ICH Q3D, as well as FDA regulations. Reliable and user-friendly testing solutions help QA/QC labs deliver precise trace elemental analyses while meeting throughput demands and data security requirements.