Measurement of the Dynamic Rates of Association and Dissociation of EGF with its Cell-surface Receptor in Intact Cells
Many important biological signals are triggered by the binding of a peptide hormone to its cognate receptor at the cell surface. Using stopped-flow fluorescence spectroscopy, the authors have been able to observe, in real time, ligand binding to epidermal growth factor receptors expressed at the surface of intact cells. This method allows for the measurement of kinetic association and dissociation rates with high data density in a native cellular environment, providing insights into the signal-initiation process in this system that have not been revealed through the determination of ligand-binding constants obtained by more traditional methods.
Binding interactions between molecules can be detected through the use of techniques such as enzyme linked immunosorbent assay (ELISA) and immunoprecipitation, providing general information about the presence or absence of an association. More accurate determinations of binding affinity or specificity include methods, such as equilibrium-binding or flow cytometry-based assays, that employ radiolabeled or fluorescently tagged ligands. Although the importance of these techniques cannot be underestimated, they can be limited in the information they provide. For example, the chemical concentration of a radiolabeled ligand can be difficult to determine, and samples often are heterogeneous mixtures of numerous mono- and di-iodinated species. The use of a fluorescently labeled ligand in flow cytometry–based assays (1) avoids many problems associated with the use of radioisotopes, but does not circumvent the limitations of approach to equilibrium methods. The product of all equilibrium-binding methods is the determination of a dissociation constant (KD) (or its inverse, an association constant, KA). While the KD (or KA) is a ratio of the rates of ligand association (kon) and dissociation (koff), those kinetic rate constants are inaccessible to equilibrium methods. Detection of these individual kinetic rate constants provides vital insights into a binding interaction — kon is the rate of ligand capture, the limiting rate constant for signal initiation, and the critical binding constant if the complex is internalized or degraded more rapidly than ligand dissociates, while koff might correlate to the duration of a signal produced by the bound complex.
Surface plasmon resonance (SPR) can provide dynamic on and off rates, and this method achieves real-time measurements with a high sampling rate (2). However, the parameters obtained from this in vitro system for ligand–receptor interactions can vary as much as three orders of magnitude from the parameters obtained using intact cells. This could reflect inaccuracies of approximating true physiological conditions or technical problems, such as unstirred layers, associated with this method. Thus, SPR is useful mainly for determination of relative ligand–receptor binding parameters (3). The method that we have developed, employing stopped-flow fluorescence spectroscopy, measures dynamic ligand-binding rates in intact cells, much more closely approximating an in vivo situation (4). Furthermore, this method is applicable to experiments in which normal ligand–receptor dynamics are disturbed indirectly through perturbations of cellular physiology.
Our use of this technique has focused upon studies of the epidermal growth factor (EGF) receptor and its cognate ligand, EGF. EGF, a small polypeptide hormone, is the archetype for a family of mitogenic hormones that bind and activate cell-surface receptors of the ErbB family (in which the EGF receptor is ErbB1) (5–7). This system has long served as a model for ligand-activated signaling networks and receptor tyrosine kinase signaling. Binding of the ligand to the receptor ultimately leads to many diverse cellular and physiological changes, including growth and proliferation, and disregulation of the EGF/ErbB signaling network has been implicated repeatedly in progression of many cancers. Because the first level of signal regulation is the interaction between ligand and receptor, association and dissociation kinetics can be critical to the ultimate strength and duration of the signal. In addition, insight into the dynamic structures of these receptors in a physiological setting can be reflected in the kinetic constants recovered by our method for studying ligand binding.
Methods
The fluorescence anisotropy binding assays that we have used to monitor association (or dissociation) of a fluorescently labeled ligand with receptors expressed in intact cells in real time have been described (4, 8). Critical needs for this assay include a well-characterized fluorescent ligand, cells expressing the receptor that are capable of surviving rapid mixing, and an instrumental set-up that includes a stopped-flow mixer, a fluorescence excitation source, and fluorescence-detection equipment.
Our studies have employed fluorescein-labeled murine EGF mutant H22Y, in which His 22 is replaced by Tyr, the homologous residue in the human hormone. It is important that a preparation of fluorescently labeled protein used for spectroscopic studies be homogeneous, have well-characterized physical properties, and that its biological activity be comparable to that of the unlabeled protein (4, 9). Moreover, the fluorophore should be singly conjugated to the ligand to avoid ambiguities with interpretation of the data, for example, distinguishing between multiple affinity states of the receptor and multiple ligand variants binding to a single receptor state. We verify these properties of the fluorescent derivative routinely in our laboratory using high performance liquid chromatography (HPLC) and mass spectrometry.
We have found that some cells of a hematopoietic origin are capable of surviving the shear forces associated with stopped flow mixing (4). For measurements on intact cells, we have used the 32D Clone 3 cell line (a pro-B cell) for this reason, and also because this cell line does not express any of the ErbB family of receptors and can be transfected to create stable cell lines expressing one or more specific members of the ErbB family. After transfection with the DNA encoding an appropriate receptor construct, the cells are sorted by fluorescence-activated cell sorting (FACS) to create monoclonal cell lines, ensuring homogeneity in receptor concentration for ligand-binding assays. Typically, we have used cell lines that express relatively high numbers of receptors (2.5 X 105 – 1 X 106 receptors/cell) to increase the sensitivity of our assay by maintaining a high effective receptor concentration in the sample (~2–10 nM) while keeping the cell density as low as possible to avoid light-scattering artifacts. All experiments have been performed at 20 °C, a temperature that prevents internalization of receptors in these cells (4).
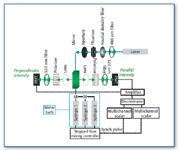
Figure 1. Schematic representation of the stopped-flow fluorescence instrument. The laser excitation source used was either a Coherent model 310 or Melles Griot model IMA 420 argon-ion laser. The stopped-flow mixing unit was a Biologic SFM-4 stopped-flow mixing unit (distributed by Molecular Kinetics); optical components including filters, lenses and polarizers were from Oriel (8). Each detection channel included an R-928 photomultiplier tube (Hammamatsu) operated in single photon counting mode. The detected signals were amplified using an SR445 DC-300 amplifier (Stanford Research), and discriminated using an SR400 two-channel photon counter (Stanford Research). Discriminator output for each channel was detected using an MCS-II multi-channel scalar board (Canberra) mounted in an Intel 486DX-based microcomputer. Data acquisition by the MCS-II boards was synchronized with the stopped-flow unit by the external synchronization output of the SFM-4 controller unit.
For an association experiment, receptor-expressing cells and fluorescently labeled ligand are loaded into separate syringes of the stopped-flow mixer, then mixed rapidly (approximately 50 ms dwell time) and injected into the fluorescence cuvette, where the fluorophore undergoes excitation. The apparatus for this experiment, depicted schematically in Figure 1, has been described previously (8). We have used polarized laser excitation because of its narrow bandwidth and intensity at a given wavelength, thus avoiding light-scattering artifacts from the cells. Fluorescence detection was set up in T-format in order to detect the emitted fluorescence that is parallel and perpendicular to the excitation polarization, and the anisotropy of the ligand can be calculated from the resulting data. The anisotropy of the fluorescent ligand reflects changes in its rotational motion, and so the binding of a fluorescently labeled EGF molecule (6 kDa) to the EGF receptor (180 kDa and embedded in the cell membrane) results in a decrease in the rotational motion of ligand, which is quantified as an increase in anisotropy. After rapidly mixing fluorescently labeled ligand with cells expressing the receptor, the measured anisotropy approaches over time the value obtained at equilibrium. The association experiment uses 15–24 combinations of ligand and receptor concentrations, each the average of ~10 separate experiments, each of which yields ~ 8 X 103 data points. The range of concentrations of ligand and receptor used in association experiments is chosen such that no experiment is limited by signal/noise while keeping ligand concentration in a physiological range. An example data set is shown in Figure 2a.

Figure 2. (a) Example data set for association experiments. The twelve curves shown represent 12 of the 24 different combinations of ligand (F-EGF) concentrations and cell densities collected in this experiment (11). Each data point shown is the average value of 8â10 repeats; only 10% of the averaged points determined for each condition are shown. The solid curves in red are the fits determined by global analysis of the full data set. (b) Example data set for dissociation experiments. The three curves shown represent 1:50, 1:75, and 1:100 dilutions of the same mixture of preequilibrated F-EGF (150 nM) and N579Q-EGF receptor expressing cells (120 X 106 cells/mL, corresponding to a receptor concentration of ~85 nM). An additional three conditions were added to the final dissociation data set that included dilutions of a different concentration of ligand and receptor (11). Each data point shown is the average value of 8â10 repeats; only 20% of the averaged points determined for each condition are shown. The solid curves in red are the fits determined by global analysis of the full data set.
To measure dissociation, a pre-equilibrated mixture of cells and fluorescent ligand is diluted with a given volume of buffer, and the anisotropy of the ligand decreases as it dissociates from the receptors (an example data set is shown in Figure 2b). There are practical limits to the dilution that can be attained experimentally, because the greater the dilution, the lower the fluorescence signal from the sample. We have found that with fluorescein-labeled derivatives and the cell densities we use typically (<1 X 108 cells/mL), a 1:100 dilution is near the limit of our detection. An alternate approach to determining dissociation rates is by a chase experiment in which receptor-expressing cells that have been pre-equilibrated with fluorescently labeled ligand are mixed with a large excess of unlabeled ligand, effectively replacing the prebound ligand as it dissociates. We find the dilution experiment preferable because it allows us to fit dissociation data and association data simultaneously to recover global parameters. In total, four to six averaged dilution experiments in combination with the association data set give a data surface that contains more than 2 X 106 real-time observations of binding interactions.
The entire data surface is globally fit (10) to the same parameters in order to obtain well-defined constants for each kinetic parameter. We fit our data to a model widely used by researchers working with the EGF receptor system, the two-independent receptor class model, which is described by:

where F-EGF is the concentration of fluorescent ligand, kon1 and koff1 are kinetic association and dissociation rates for one receptor class (EGFR1), and kon2 and koff2 are the corresponding kinetic rates for the second class of receptors (EGFR2). The receptor classes defined by this model might or might not have a relationship to specific structural states of the EGF receptor. The observed anisotropy is calculated from the concentrations of the individual species using the formula
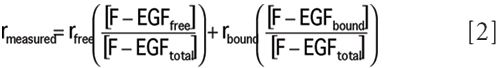
where rfree is the anisotropy of F-EGF free in solution and rbound is the anisotropy of F-EGF bound to receptors in cells. Parameters recovered from this analysis are rbound, the effective receptor concentration at each cell density used in the experiments, association rates, dissociation rates, and the fraction of receptors in each receptor class. In addition, error analysis is performed to obtain 67% confidence intervals (standard deviations) for each parameter (10).
Results
We have used this biophysical technique to perform a series of stopped-flow fluorescence experiments using fluorescein-labeled H22Y-mEGF (1) and cells expressing various receptor constructs in order to measure explicitly the kinetic association and dissociation rates of ligand with receptor in living cells. Figure 3 shows our results for cells expressing wild-type EGF receptor (LE1.15) (4), N579Q-EGF receptor (11), a construct that mimics a glycosylation variant of the wild-type EGF receptor found in at least one tumor cell line that overexpresses the EGF receptor (12), and wild-type EGF receptor coexpressed with ErbB2 (13). The effect of ErbB2 expression on EGF binding to the EGF receptor is exemplified by two cases, one in which the level of ErbB2 expression is ~25% that of EGF receptor expression (L1-21.3) and one in which the level of ErbB2 expression is ~80% that of the EGF receptor expression (L1-2.2 cells).

Figure 3. Results of experiments performed on 32D cells expressing EGF receptors only (LE1.15) (4), N579Q-EGF receptors (11), or both the EGF receptor and ErbB2 in varying ratios (13). The values of k off, k on, and K D for each affinity state, as well as the fraction of receptors in each state (solid versus hatched bars) are shown. Error bars represent 67% confidence intervals.
The calculated KD values for the two affinity states (defined in Equation 1) are very similar to one another across cell lines, with most observed differences not significant statistically. However, explicit determination of the values for kon and koff reveals that even though the equilibrium dissociation constants are similar, the two affinity states observed for the EGF receptor expressed in the presence of ErbB2 or expressed without glycosylation at Asn-579 are distinct kinetically from those of the wild-type EGF receptor. Thus, in the wild-type EGF receptor population, the high-affinity class is defined by a faster rate of ligand capture (kon1) than the low affinity class. In contrast, the high-affinity class of either EGF receptors in the presence of ErbB2 or the glycosylation mutant N579Q-EGF receptor is characterized by the very slow rate of ligand release (koff1). This key difference in the character of the high affinity state had not been detected by previous methods.
Interpretation of recovered kinetic parameters in the context of any model must be done carefully, but this switch in the character of high-affinity binding sites is notable, and likely reflects different conformations of the EGF receptor. In the context of recent structural insights and the proposal of a conformational model of ligand binding to and activation of the EGF receptor (Figure 4 and references 7 and 14), the parameters that we recovered from these ligand-binding experiments might be correlated with structural transitions of the EGF receptor. The wild-type EGF receptor is thought to be mostly in an auto-inhibited or tethered conformation before ligand binding (6, 7, 14). In contrast, we have proposed that the N579Q-EGF receptor glycosylation mutant spends more time sampling an untethered state prior to ligand binding (11). Ligand binding also has been proposed to be affected by dimerization of receptors, and in this context, because ErbB2 is a preferred dimerization partner for the EGF receptor, our analysis suggests that the presence of ErbB2 has shifted the EGF receptor to an untethered or pre-dimerized state. It is possible that different conformations of the receptor produce different signals. In this respect, we have found that the ligand-bound N579Q-EGF receptors produce different downstream signals than wild-type EGF receptors. The pattern of EGF-stimulated tyrosine-phosphorylated proteins in cells expressing N579Q-EGF receptors is significantly different than the pattern of tyrosine-phosphorylated proteins obtained from cells expressing WT-EGF receptors (11). In addition, 32D cells expressing N579Q-EGF receptors do not survive in a serum-rich medium in the absence of interleukin-3 (a cytokine necessary for survival and proliferation of parental 32D cells) (11), the same conditions under which 32D cells expressing wild-type EGF receptors are viable (15).
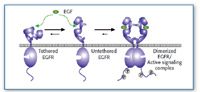
Figure 4. Proposed model for activation of the EGF receptor, based upon the structural studies of the ectodomain (7, 14). Ligand can bind either to a tethered or untethered receptor, which induces dimerization (or to a pre-formed dimer), resulting in a fully active receptor capable of signaling. In the absence of ligand, wild-type EGF receptors are thought to be mostly tethered (7, 14), and N579Q-EGF receptors appear to spend more time in an untethered conformation (11). EGF receptor dimers can be either homodimers or heterodimers of the EGF receptor, for example, with ErbB2. Structural transitions in the ectodomain might correlate with structural transitions in the intracellular kinase domain to affect different downstream signals.
Conclusion
We have found this method for determining kinetic association and dissociation rates for the interaction of a hormone with its cell-surface receptor in living cells capable of revealing previously unreported differences in the character of the hormone's binding site under different physiological conditions. Further, our studies have shown that these differences in binding states correlate with observable differences in downstream signaling. Though not a high-throughput method, at the present time, it is the only technique that can provide measurement of the dynamic rates of ligand association and dissociation to a receptor in an intact cell. Thus, the technique could be critical in determining the functional nature of ligand–receptor interactions and their modulation by factors that perturb cellular physiology. This method should be applicable to other ligand–cell-surface receptor systems in which a singly fluorescently tagged ligand of high purity can be produced and the receptor can be expressed in shear-resistant cells.
Acknowledgements
This work was supported by a grant R01 GM055056 (A.H.B. and J.V.S.) from the National Institutes of Health, Bethesda, MD.
Kristin B. Whitson and Albert H. Beth are with Vanderbilt University's School of Medicine, molecular physiology and biophysics dept. (Nashville, TN). James V. Staros is a professor in the department of biochemistry and cell biology at SUNY-Stony Brook (New York). E-mail: james.staros@stonybrook.edu
References
1. R.A. Stein, J.C. Wilkinson, C.A. Guyer, and J.V. Staros, Biochemistry 40, 6142–54 (2001).
2. T. Chen, N. MacDonald, and J. Wingard, PharmaGenomics, 44–47 June 2003.
3. D. J. O'Shannessy and D.J. Winzor, Anal. Biochem . 236, 275–283 (1996).
4. J.C. Wilkinson, R.A. Stein, C.A. Guyer, J.M. Beechem, and J.V. Staros, Biochemistry 40, 10230–42 (2001).
5. R.A. Stein and J.V. Staros, J. Mol. Evol. 50, 397–412 (2000).
6. R.N. Jorissen, F. Walker, N. Pouliot, T.P. Garrett, C.W. Ward, and A.W. Burgess, Exp. Cell Res. 284, 31–53 (2003).
7. A.W. Burgess, H.S. Cho, C. Eigenbrot, K. M. Ferguson, T.P. Garrett, D.J. Leahy, M.A. Lemmon, M.X. Sliwkowski, C.W. Ward, and S. Yokoyama, Mol. Cell 12, 541–552 (2003).
8. J.C. Wilkinson, J.M. Beechem, and J.V. Staros, J. Recept. Signal Transduct. Res. 22, 357–371 (2002).
9. K.B. Whitson, J.M. Beechem, A.H. Beth, and J.V. Staros, Anal. Biochem . 324, 227–236 (2004).
10. J.M. Beechem, Methods Enzymol. 210, 37–54 (1992).
11. K.B. Whitson, S.R. Whitson, M.L. Red-Brewer, A.J. McCoy, A.A. Vitali, F. Walker, T.G. Johns, A.H. Beth, and J.V. Staros, Biochemistry 44, 14920–14931 (2005).
12. Y.Zhen, R.M. Caprioli, and J.V. Staros, Biochemistry 42, 5478–92 (2003).
13. J.C. Wilkinson and J.V. Staros, Biochemistry 41, 8–14 (2002).
14. K.M. Ferguson, M.B. Berger, J.M. Mendrola, H.S. Cho, D.J. Leahy, and M.A. Lemmon, Mol. Cell. 11, 507–517 (2003).
15. J.A. Ewald, J.C. Wilkinson, C.A. Guyer, and J.V. Staros, Exp. Cell Res . 282, 121–131 (2003).

New SERS Platform Enhances Real-Time Detection of Cardiovascular Drugs in Blood
May 13th 2025Researchers at Harbin Medical University recently developed a SERS-based diagnostic platform that uses DNA-driven “molecular hooks” and AI analysis to enable real-time detection of cardiovascular drugs in blood while eliminating interference from larger biomolecules.
The Role of LIBS in ChemCam and SuperCam: An Interview with Kelsey Williams, Part III
May 2nd 2025In this extended Q&A interview, we sit down with Kelsey Williams, a postdoctoral researcher at Los Alamos National Laboratory (LANL), who is working on planetary instrumentation using spectroscopic techniques such as laser-induced breakdown spectroscopy (LIBS) and laser ablation molecular isotopic spectrometry (LAMIS). In Part III, Williams goes into detail about ChemCam and SuperCam and how LIBS is used in both these instruments.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)
















