ICP-MS Speciation Analysis: Three Roles of Selenium
Spectroscopy
The element selenium plays three distinct roles in biological processes, functioning in turn as a toxicant, a chemopreventive agent, and a heavy metal antagonist. This article discusses current research associated with each role, and how ICP-MS can be employed to better understand and utilize selenium's properties.

For decades, selenium has been considered an essential element to human nutrition. It plays an important role in many biological processes, generally exerting its biological affect through selenoproteins. Selenium is incorporated specifically into proteins by a UGA codon that encodes for selenocysteine. There are over 30 known selenoproteins in mammalian systems, including a number of glutathionine peroxidases (GPx), iodothyronine 50-deiodinases (IDI), sperm capsule selenoprotein, and thioredoxin reductase (1, 2). Selenium also is required for the production of triiodothyronine, a thyroid hormone necessary for healthy brain and bone development, normal growth, and thermoregulation (3). Proper immune function also is selenium-dependent, which recently has prompted investigations into its ability to inhibit HIV and AIDS progression (4).
Much of the recent research on selenium is accomplished with inductively coupled plasma–mass spectrometry (ICP-MS). This method has proved to be sensitive, robust, and versatile enough to study selenium and selenium species in a multitude of situations. Although Ar–Ar dimers created by the plasma can act as an interference at m/z 80, the major selenium isotope, selenium still is detected easily and quantified by monitoring its other isotopes at m/z 77, 78, or 82. The use of collision cell technology, however, allows for the removal of the Ar–Ar interference, thus allowing for improved selenium detection. In this system, helium or hydrogen gas is introduced that bombards the dimers, thereby breaking them apart so that they no longer interfere at m/z 80. Many different liquid chromatography (LC) techniques are commonly coupled to ICP-MS for selenium speciation. These methods have allowed for the identification of specific selenium species of biological importance. Further confirmation of these species often is achieved through electrospray ionization MS (ESI).
As more research is conducted on selenium and selenium-containing compounds, the diversity of this element's various species becomes more apparent. Not only is selenium a known nutrient, it also can be a toxicant at even slightly elevated levels. Selenosis, or the poisoning caused by selenium, is characterized by discoloration of the skin and nails, gastrointestinal disturbances, fatigue, hair loss, nervous system abnormalities, and garlic-scented breath. Because high levels of selenium in the environment can have adverse effects on plant and wildlife, selenium remediation has become an issue that currently is being addressed in many areas of the world.
Ironically, even though selenium itself can be toxic, normal levels of selenium in blood plasma actually can reduce the toxicity of other heavy metals through the formation of equimolar complexes. These complexes follow different biological pathways than the free heavy metal, thereby reducing the potential damage caused by the metal as well as that of the selenium itself.
Despite the fact that extreme levels of selenium can prove toxic, it has been found that the consumption of slightly elevated levels of selenium actually can be quite beneficial. Certain selenium species, namely selenomethionine and methylselenocysteine, have exhibited chemopreventive properties, particularly towards prostate cancer. As a result, selenium speciation of many foods and nutritional supplements has become increasingly worthwhile.
This article will focus on three very distinct roles that selenium can play in biological processes: as a toxicant, a chemopreventive agent, and a heavy metal antagonist. By discussing the current research associated with each, it will become apparent how ICP-MS can be employed in a multitude of capacities to better understand and better utilize these selenium properties.
Selenium as a Toxicant
Although selenium is an essential nutrient, it can be toxic at relatively low levels. While the USDA recommends 70 mg Se per day for human health, roughly 800 mg daily can lead to selenosis. In 1983, the symptoms associated with selenosis were seen in many of the animals of the Kesterson National Wildlife Refuge in California. The oddly shaped beaks, missing wings, and twisted legs of newborn birds in the area prompted scientists to discover unusually high levels of selenium had accumulated in the Kesterson Reservoir water. It was determined that this had been caused by irrigation systems designed to support agriculture on saline soils. These systems flushed large amounts of water through the area, which drained into the Kesterson Reservoir, carrying with them naturally occurring levels of selenium salts. The accumulation of this selenium proved to have a toxic effect on many of animals as well as much of the plant life in the area.
The evaporation of the selenium-polluted water has led to an even greater accumulation of selenium in the soil. Variations in organism tolerances to selenium have led the investigation of phytoremediation as a possible remedy to selenium contamination. For phytoremediation to be effective, a plant must be used that is capable of accumulating anywhere from hundreds to tens of thousands of micrograms per gram of selenium; is sufficiently robust to give maximal accumulation; and produces a large biomass. Brassica juncea has been found to meet these criteria and has been studied fairly extensively in recent years as a selenium phytoremediator.
Brassica juncea is naturally a selenium accumulating plant, and has been studied fairly extensively as a possible phytoremediator. A significant portion of the selenium incorporated into the plant eventually is volatized back into the environment, as depicted in Figure 1. The species formed by the phytovolatization process are primarily dimethylselenide (DMSe) and dimethyldiselenide (DMDSe), which are 500–700 times less toxic than the inorganic species typically found in selenium-contaminated soils (5, 6). The complete mechanism involved in the volatilization process has not yet been fully confirmed, but it is believed to take place through the formation of selenonium ions that are cleaved to DMSe analogous to the sulfonium assimilation pathway (5, 7).

Figure 1. Phytoremediation pathway of selenium accumulator Brassica juncea.
However, genetically modified versions of Brassica juncea have been introduced that allow for the production of selenium volatiles by different mechanisms. Incorporation of an Se-cysteine methyltransferase (SMT) gene in the plant causes incorporated selenium to be converted to methylselenocysteine (MSC), which then is metabolized into DMDSe (8). The presence of MSC in these genetically modified plants has been confirmed by LC-ICP-MS, as shown in Figure 2 (9). Because it is possible that MSC is a precursor to a chemopreventive species, this particular genetic modification would not only be useful in detoxifying the selenium in contaminated soils, but also might produce plants with added nutritional benefit.
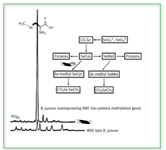
Figure 2. Difference between Se(methyl)-Se-cysteine production in wild type versus SMT genetically modified Brassica juncea. (from ref. 19, with permission).
Selenium as a Chemopreventive Agent
In 1996, Clark and colleagues conducted a study to determine whether a nutritional supplement of selenium would decrease the incidence of cancer (10). Over 1300 participants were treated with either a placebo or 200 µg/day Se for a period of 4.5 years. Those patients receiving the selenium supplementation exhibited a 39% reduction in carcinoma incidence, namely colon and prostate, as well as a 48% decrease in mortality. Since then, much research has centered on the potential of Se in cancer prevention (11–17). Based upon the findings of these studies, the National Cancer Institute initiated a 12-year study in 2001 to investigate the long-term effects of selenium and vitamin E on prostate cancer (18). The Selenium and Vitamin E Chemoprevention Trial (SELECT) will cost over $100 million and will involve more than 32,000 men over 55 years of age, making it the largest cancer chemoprevention study ever.
The selenium species of most interest in cancer prevention has been selenomethionine, due to its suggested conversion to methylselenol in humans. Studies have indicated that methylselenol actually might be the critical metabolite in selenium chemoprevention, but due to its high reactivity, this compound cannot be investigated directly (3, 19). However, it has been suggested that methylselenocysteine (MSC) is converted to methylselenol more directly and therefore, more efficiently, than selenomethionine. Consequently, MSC has been proven to be twice as active as selenomethionine in suppressing some forms of carcinogenesis (19).
As indicated in the previous discussion, MSC can be produced in genetically modified plants, namely Brassica juncea. However, this compound also can be produced by wild type varieties of other plants, and more importantly, vegetables, which humans can consume to obtain chemopreventive benefits. Specifically, enzymatic extracts of both onions and garlic bulbs have been found to contain both selenomethionine as well as γ-glutamyl-methylselenocysteine, the carrier of MSC (20). Recent work has focused on the enrichment of green onions (Allium fistulosum), which consists mostly of leaf rather than bulb, because they are used widely and eaten in toto. After supplementing these plants with selenite, the coupling of size exclusion chromatography (SEC) as well as reversed-phase chromatography to ICP-MS were used to detect the presence of selenomethionine and MSC in the green onion samples. The presence of γ-glutamyl-methylselenocysteine was confirmed further with ESI-MS (21).
Selenomethionine and MSC also have been detected in several varieties of mushrooms. Mushrooms have been a part of the human diet for thousands of years and their consumption continues to increase worldwide. Not only are edible mushrooms good sources of various mineral elements, they also are known to be selenium accumulators (22). Both shiitake (Lentinula edodes) and crimini (Agaricus bisporus) varieties of mushrooms have been enriched with selenium and evaluated with SEC-ICP-MS and reversed phase LC-ICP-MS to reveal the presence of these cancer chemopreventive agents (1).
Selenium as a Heavy Metal Antagonist
In vivo studies have shown that transition metal ions can interact with selenium to form equimolar complexes, which can bind selectively to plasma proteins, thereby reducing the toxicity of the transition metal as well as the selenium. Although detoxification of silver and cadmium also is known to occur through interaction with selenium, the mechanism is not as completely understood as that of mercury (23). When selenite is co-administered with mercury in the bloodstream, the selenium species is taken up selectively by red blood cells and reduced by glutathione (GSH). This reduced form of selenium complexes with the mercury in the plasma to form a compound comprised of the two elements in an equimolar ratio (Hg–Se). It is indicated that this compound then selectively binds to Selenoprotein P to form a (Hg–Se)-Sel P complex (23–25). The formation of this complex changes the distribution and localization of Hg in organs and at the subcellular level dramatically from that which is reported when Se is absent. Therefore, it seems that the complexing of selenium with mercury results in the redirection of mercury in biological pathways, thereby inhibiting the element's toxicity (24).
Current research in our labs has utilized SEC coupled to ICP-MS to further study the formation of Hg–Se complexes in various plant species. In these studies, both soybeans and Brassica juncea were enriched with selenium as well as with both mercury and selenium simultaneously. It was found that the presence of mercury did not alter the selenium species produced in the plants, but caused more selenium to be incorporated. The assimilation of mercury into the plants appeared to involve high molecular weight species that also included selenium and appeared only in the roots. Little was translocated into the stems, leaves, or bean. The chromatograms in Figure 3, which correspond to Brassica juncea, indicate that the plant might interdict the Hg flow to animals by sequestering it in a plant compartment in the roots (26).
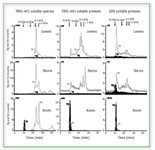
Figure 3. 80Se and 202Hg SEC/ICP-MS chromatograms of the three compartments of Brassica juncea previously supplemented with SeO32- and Hg(II). (from ref. 26, with permission).
Conclusion
As more research is conducted, more is learned about the various roles that selenium can play in the biological processes of various organisms. Not only is this element known to be essential to basic human health, it is becoming increasingly apparent that it might be important for reasons other than nutrition. The toxic effects of heavy metals can be reduced when they complex with plasma selenium. These equimolar selenium and metal species allow for the relocalization and consequent detoxification of the metal. Specific forms of selenium have exhibited chemopreventive properties toward various forms of cancer. While the exact mechanisms by which selenomethionine and methylselenocysteine affect carcinogenesis still are being elucidated, the production of these species in edible plants is being exploited. Yet it is important to remember that not only are the benefits of selenium associated with specific chemical species, but also with specific quantities. The crisis experienced at the Kesterson Wildlife Refugee demonstrated the toxic effect that high levels of selenium can have on various organisms. These three faces of selenium — as toxicant, as chemopreventive agent, and as heavy metal antagonist — represent the complicated and delicate biochemical nature of elemental species, a nature that scientists are striving continually to better understand.
Katie DeNicola Cafferky, Douglas D. Richardson, and Joseph A. Caruso are with the University of Cincinnati, Department of Chemistry, Cincinnati, OH. E-mail: joseph.caruso@uc.edu
References
1. V. Gergely, K. M. Kubachka, S. Mounicou, P. Fodor, and J.A. Caruso, J. Chromatogr., A; submitted for publication.
2. D.H. Holben, A.M. Smith, J. Am. Diet. Assoc. 99, 836–843 (1999).
3. C. Ip, J. Nutr. 128, 1845–1854 (1998).
4. N. Terry, A.M. Zayed, M.P. DeSouza, and A.S. Tarun, Ann. Rev. Plant Phys. Mol. Biol. 51, 401–432 (2000).
5. T.D. Grant, M. Montes-Bayon, D. LeDuc, M.W. Fricke, N. Terry, and J.A. Caruso, J. Chromatogr., A 1026, 159–166 (2004).
6. J.H. Ansede, P.J. Pellechia, and D.C. Yoch, Environ. Sci. Technol. 33, 2064–2069 (1999).
7. T.D. Colmer, F. Corradini, G.R. Cawthray, and M.L. Otte, Phytochem. Anal. 11, 163–168 (2000).
8. N. Terry, A.M. Zayed, M.P. DeSouza, and A.S. Tarun, Annu. Rev. Plant Physiol. Plant Mol. Biol. 51, 401–431 (2000).
9. J. Meija, M. Montes-Bayon, J.A. Caruso, and A. Sanz-Medel, Trends in Anal. Chem.; accepted for publication.
10. L.C. Clark et al., JAMA 276, 1957–1963 (1996).
11. K. El-Bayoumy, Drugs of the Future 22, 539–545 (1997).
12. G.F. Combs and W.P. Gray, Pharmacol. Therapeutics 79, 179–192 (1998).
13. S.D. Cho et al., J. Mol. Cancer Therapeutics 3, 605–612 (2004).
14. Y. Dong, H.E. Ganther, C. Stewart, and C. Ip, Cancer Res. 62, 708–714 (2002).
15. C. Ip, K. El-Bayoumy, P. Upadhyaya, H.E. Ganther, S. Vadhanavikit, and H. Thompson, Carcinogenesis 15, 187–192 (1994).
16. J. Lu and C. Jiang, Nutrition and Cancer 40, 64–73 (2001).
17. V. Venkateswaran, N.E. Fleshner, and L.H. Klotz, Prostate Cancer and Prostatic Diseases 7, 54–56 (2004).
18. L. DeFrancesco and M. Cimons, Nat. Med. 7, 1076 (2001).
19. C. Ip, Y. Dong, and H.E. Ganther, Cancer Metastasis Rev. 21, 281–289 (2002).
20. H. Ge, Z.J. Cai, J. Tyson, P.C. Uden, E.R. Denoyer, and E. Block, Anal. Commun. 33, 279–281 (1996).
21. M. Shah, S. Kannamkumarath, J.C.A. Wuilloud, R.G. Wuilloud, and J.A. Caruso, J. Anal. At. Spectrom. 19, 381–386 (2004).
22. Y. Ogra, K. Ishiwata, J.R. Encinar, R. Lobinski, and K.T. Suzuki, Anal. Bioanal. Chem. 379, 861–866 (2004).
23. C. Sasakura and K.T. Suzuki, J. Inorg. Biochem. 71, 159–162 (1998).
24. S. Yoneda and K.T. Suzuki, Toxicol. Appl. Pharmacol . 143, 274–280 (1997).
25. S. Yoneda and K.T. Suzuki, Biochem. Biophys. Res. Commun . 231, 7–11 (1997).
26. S. Mounicou, M. Shah, J. Meija, J.A. Caruso, A.P. Vonderheide, and J. Shann, J. Anal. At. Spectrom.; submitted for publication.

High-Speed Laser MS for Precise, Prep-Free Environmental Particle Tracking
April 21st 2025Scientists at Oak Ridge National Laboratory have demonstrated that a fast, laser-based mass spectrometry method—LA-ICP-TOF-MS—can accurately detect and identify airborne environmental particles, including toxic metal particles like ruthenium, without the need for complex sample preparation. The work offers a breakthrough in rapid, high-resolution analysis of environmental pollutants.
Advanced Raman Spectroscopy Method Boosts Precision in Drug Component Detection
April 7th 2025Researchers in China have developed a rapid, non-destructive Raman spectroscopy method that accurately detects active components in complex drug formulations by combining advanced algorithms to eliminate noise and fluorescence interference.