Influence of Na+ and K+ Concentration in solvents on Mass Spectra of Peptides in LC–ESI-MS
The concentration dependent influence of Na+ and K+ions on mass spectra of peptides is shown with human gastrin as a model peptide. With electrospray ionization the doubly charged protonated molecule ion [M+2H]2+ is normally the preferred ionization product. However, trace amounts of alkali metal ions already form clusters (adducts) with the peptide molecule, such as [M+H+Na]2+, which become dominating at higher concentrations. With Na+/K+ concentrations below 0.1 mg/kg (ppm) only a few clusters appear, which allow the correct doubly charged molecule ion to be assigned for a subsequent MS–MS experiment. With concentrations of 10 ppm and higher the alkali clusters become the most abundant peaks in the spectrum, and the absolute sensitivity is decreased by a factor of 5–10. Experiments were performed with water and water–methanol mixtures with a known Na+/K+ +content.

The effect of dispersing a solvent into charged droplets, when applying a high electric potential to an effluent capillary, was first described by Zeleny in 1917 (1). It was investigated in more detail in 1955 by Drozin (2) and used in pioneering work for an electrospray interface by Dole et al (3,4). This was continued in 1984 by Yamashita and Fenn (5,6), which finally resulted in a description of an LC–MS interface in 1985 (7). Today electrospray ionization (ESI) is the most widely used ionization technique in LC–MS. This is especially true in protein mass spectrometry, where series of multiply charged ions are observed and used to determine the molecular mass (8,9). Furthermore, after tryptic digestion, the resulting peptides can be measured with LC–ESI-MS, to obtain amino acid sequence information after a second MS/MS-step (10–14).
When characterizing peptides using LC–ESI-MS, often multiply charged ions are observed (usually doubly charged [M+2H]2+ ions in the instance of tryptic peptides). When the charge state is identified (15), the (monoisotopic) molecular mass can be calculated and the ion can be isolated to permit MS/MS for amino acid sequence information. With the growing demand for such data in bioscience (proteomics), the ability to do this in automated mode has been discussed (16–18). For this purpose, it is necessary to identify without doubt the correct doubly or multiply charged ions as precursors for MS/MS; in the ideal instance all relevant ions should occur at high abundance. However, this is not always the situation, because sometimes protonation is not the only ionization process.
Since the early days of electrospray, theories of ion formation have been discussed. The hypothesis of Dole (3), based upon the assumption that the observed ions are produced by simply desolvating the charged droplets, is called the "charge residue model". Roellgen et al (19). postulated a "soft desolvation" of ions by solvent evaporation from small charged droplets by either electrohydrodynamic or mechanical instabilities (or both). These theories led to the present model of a "coulomb explosion", where the charge stays with the analyte when a charged droplet becomes smaller and eventually "explodes", losing all neutral solvent molecules (20). To produce charged droplets in a first step it is necessary to have ions present in the effluent, which undergoes the electrospray process. To drive the ionization in the direction of the protonated molecules, it is common to add formic or acetic acid to the solvent, usually in concentrations of 0.1 to 0.5%. The protons are transferred to the analyte during the process of ionization in the electrospray interface. If the solvent contains other cations, these may compete with the protons to form adducts with the analyte. Wang and Cole (21) investigated the influence of ionic strength on the positive-ion electrospray mass spectra of myoglobin and reported a 10-fold decrease in signal intensity and almost no change of charge-state distribution, when increasing the salt concentration. This effect can be used in a positive way for some applications (22,23) and is especially helpful in MS of glycosides. However, it is normally undesirable in protein and peptide analysis because here the desired result is the formation of the doubly charged molecule ion at high abundance, which has to be identified and isolated for subsequent MS–MS analysis.
The unwanted formation of metal ion adducts is often caused by salt impurities from sample preparation or from the solvents (eluents). The aim of this work was to study the influence of the ubiquitous sodium and potassium ions, present in trace amounts in the solvent, on the electrospray mass spectra of peptides, using human gastrin as a model peptide (24,25). Water and methanol were chosen because of their protic behavior and their ability to leach Na+ and K+ from surfaces (i.e., from glass bottles used for solvent storage.)
Experimental
A stock solution of human gastrin (pGlu-Gly-Pro-Trp-Leu-Glu-Glu-Glu-Glu-Glu-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2) from Fluka (Buchs SG, Switzerland) was prepared in water (HPLC grade, Fluka) with a concentration of 100 μg/mL. Aliquots were diluted to 10 μg/mL with the respective Na+ /K+ adjusted solvent just before performing the MS experiments. The solvents with different Na+ /K+ contents were prepared by adding NaCl and KCl purris. p.a. (Fluka) to Millipore ultra-purified water (Eschborn, Germany). Water-methanol mixtures (50/50; v/v) were prepared with three selected Na+ /K+ dilutions and methanol gradient grade from Riedel-de Haën (Seelze, Germany). Formic acid purris. p.a., (Fluka) was added to each solution to a level of 0.2% prior to mass spectrometry to improve ionization efficiency by protonation.
The amounts of Na+ and K+ were measured with ICP-OES spectroscopy (TJA IRIS/AP HR DUO, Thermo Elemental, Dreieich, Germany). The LC/ESI–MS experiments were performed with a Thermo Finnigan LCQ Advantage LC–MS ion trap (Thermo Electron, Dreieich, Germany) using electrospray ionization and direct syringe infusion.

Table I. Influence of Na+/K+-concentration in LCâMS solvents on spectral abundance and sensitivity. *See comments in text.
Results and Discussion
The Na+ /K+ contents of the prepared solvents, determined by ICP-OES, are shown in Table 1. The chosen concentrations cover a range from 0.01 to 900 mg/kg (ppm). At concentrations below 0.1 ppm the preferred ionization product is [M+2H]2+ , which appears as the base peak. Sodium and potassium ions form [M+H+Na]2+ and [M+H+K]2+ adducts in low abundance (Figure 1).
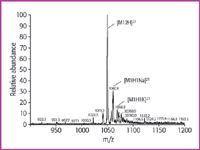
Figure 1. ESI-MS of human gastrin (M 5 2097) in solvent w2: water (0.2% formic acid) with 0.10 ppm Na+ and 0.09 ppm K+. Only few adducts are observed, the doubly charged molecule ion can be determined without doubt.
With higher concentrations of alkali ions the total ionization efficiency for the peptide and the relative abundance of [M+2H]2+ decreases, whereas both the amount and the variety of metal ion clusters increases. In solution w3 with a Na+ /K+ concentration of slightly below 1 ppm the [M+2H]2+ peak is no longer the most abundant peak in the spectrum, but with 77% is still the most abundant of the relevant group of doubly charged ions at around m/z 1050.
When the alkali ion content is near 10 ppm, the relative abundance of the doubly protonated molecule ion is decreased by a factor of 4, the total response for the peptide is decreased 10-fold, and the sodium ion adducts become dominant with [M+2Na]2+ as the most abundant ion within the doubly charged group (Figure 2). At this concentration mixed adducts containing both sodium and potassium ions can also be observed. Na+ appears to have the higher affinity for gastrin under these conditions, probably because of the ion radius. Therefore lithium might be expected to have even higher affinity but was not investigated in this study.
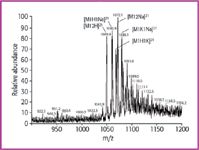
Figure 2: ESI-MS of human gastrin (M 5 2097) in solvent w4: water (0.2% formic acid) with 9.0 ppm Na+ and 8.4 ppm K+. Clusters with alkali ions are multiple and dominating. The most abundant peak is the doubly charged sodium adduct [M 1 2Na]2+, the absolute sensitivity is decreased 10-fold. The series of doubly charged ions is extended to higher masses (m/z 5 1091, 1099, 1110 etc.) caused by exchange of peptide protons (5 GLU units) against Na+ or K+.
The doubly charged ions at higher m/z values in this spectrum (m/z 1091, 1099, 1110, etc.) can be explained as an exchange of peptide protons (5 Glu residues) against Na+ or K+ , which has no influence on the charge state (2+). With many free acidic groups within the test molecule, the ability to form adducts of various kinds with Na+ and K+ is very high. So, the extent of this effect is dependent upon the respective peptide and cannot be predicted for unknown proteins/ peptides.
Figure 3 shows a whole range spectrum obtained in water with a rather high alkali ion concentration of 90 ppm. The signals in the range of the doubly charged ions are below 5% relative abundance. Signals are observed at lower m/z (555–583) that might correspond to 4+ ions with varying numbers of H+ , Na+ , and K+ , which cannot be used for further steps in amino acid sequence elucidation.
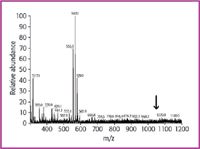
Figure 3: ESI-MS of human gastrin (M 5 2097) in solvent w5: water (0.2% formic acid) with 90 ppm Na+ and 89 ppm K+; full spectrum. No signals in the range of m/z 5 1049.9 (arrow), low mass fragments (m/z
Besides water, methanol is also a protic solvent, which could leach Na+ /K+ ions from the solvent glass bottles. Therefore, the influence of these ions was also investigated in methanol/water mixtures of different Na+ /K+ content. The chosen concentration range is much smaller (Table 1) because, as shown, very low alkali ion concentrations of about 0.1 ppm (wm1) already have a remarkable tendency to form alkali ion adducts (Figure 4). The doubly charged ion pattern is qualitatively the same as with an eight-times higher alkali ion concentration in water (Figure 2), but the relative abundances are different with [M+2H]2+ as the most abundant ion in this group; thus it is still possible to determine and select the correct mass peak for collision experiments to obtain amino acid sequence information. In a solution with 0.84 ppm Na+ the [M+2Na]2+ ion is dominant in the doubly charged group and is even the most abundant (100%) in the whole spectrum. With a Na+ concentration of 6.4 ppm and a K+ concentration of 5.6 ppm, the relative and absolute sensitivity are decreased remarkably. [M+2Na]2+ is the tallest peak in the doubly charged group (37%) and low mass ions occur at high abundance (100%), making this spectrum useless for further elucidation steps.
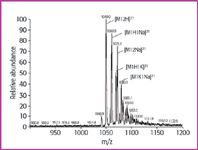
Figure 4: ESI-MS of human gastrin (M 5 2097) in solvent wm1: water-methanol (50/50; v/v) with 0.12 ppm Na+ and
Conclusion
Summarizing these results, it is evident that alkali ions, especially sodium, in trace amounts compete with protons present in much higher concentration to form adducts with the peptide; this effect will vary with different peptides. Therefore it is necessary, when doing amino acid sequencing of peptides with LC/ESI–MS, to keep the alkali metal ion content of solvents and samples as low as possible (desalting). Even ultra-pure solvents can be contaminated, when stored in glass bottles, which are not coated or surface treated. This is especially true for protic solvents such as water and methanol. Acetonitrile usually has minor problems with alkali ion load. Three tested samples did not show any alkali ion adducts such as those discussed above with water or methanol, and the content of alkali ions was very low in the ppb range. Lithium is expected to have an even stronger effect than sodium but was not investigated in this study, because it is by far less ubiquitous than sodium and potassium, which are responsible for most such problems in LC/ESI–MS. Finally it should be noted that the test peptide used here, with five acidic residues (Glu), is particularly susceptible to formation of multiple adducts with alkai metal ions, both as ionizing agents and as replacements for the acidic protons. The relative yields of protonated versus sodiated species can also vary with the geometry of the ion source, and can be minimized by adjusting various source parameters, but in general the effect is present for every instrument and almost any peptide; only the extent will be different. However, the more sensitive a measurement is, the more influence this effect will have. In this instance it is even more important to take care of good sample clean-up and solvents, which are free of alkali impurities.
Joachim Emmert is an analytical chemist. He obtained his Ph.D. in chemistry in 1982 at the University of Heidelberg, Germany. He was involved in applications and method development of Hewlett-Packard and Boehringer Mannheim. Since 2003 he has been responsible for MS applications development within the Sigma-Aldrich Company of Fluka, Switzerland.
Markus Pfluger obtained his MSc degree in analytical chemistry in 1996. His diploma thesis was about isotope dilution by means of high resolution ICP-MS. He has been working in the trace analytical department of Fluka since 2000.
Fabian Wahl received his PhD in organic chemistry and biochemistry in 1993 in Freiburg with Professor Dr Horst Prinzbach. Since then he has served in different positions within Fluka as a part of Sigma-Aldrich, being responsible as Manager for R&D Europe since 2000.
References
1. J. Zeleny, Phys. Rev. 10, 1 (1917).
2. V.G. Drozin, J. Colloid Sci. 10, 158 (1955).
3. M. Dole et al., J. Chem. Phys. 49, 2240 (1968).
4. J. Gieniec et al., Biomed. Mass Spectrom. 11, 259 (1984).
5. M. Yamashita and J.B. Fenn, J. Phys. Chem. 88, 4451 (1984).
6. M. Yamashita and J.B. Fenn, J. Phys. Chem. 88, 4671 (1984).
7. C.M. Whitehouse et al., Anal. Chem. 57, 675 (1985).
8. T.R. Covey et al., Rapid Commun. Mass Spectrom. 2, 249 (1988).
9. M. Mann, C.K. Meng and J.B. Fenn, Anal. Chem. 61, 1702 (1989).
10. M.J. Huddleston, M.F. Bean and S.A. Carr, Anal. Chem. 65, 877 (1993).
11. J. Ding, W. Burkhart and D.B. Kassel, Rapid Commun. Mass Spectrom. 8, 94 (1994).
12. J.A. Taylor, K.A. Walsh and R.S. Johnson, Rapid Commun. Mass Spectrom. 10, 679 (1996).
13. K. Fujii et al., Anal. Chem. 69, 5146 (1997).
14. T.A. Fligge, K. Bruns and M. Przybylski, J. Chromatogr., B: Biomed. Appl. 706, 91 (1998).
15. G. Neubauer and R.J. Anderegg, Anal. Chem. 66, 1056 (1994).
16. R. Bonner and B. Shushan, Rapid Commun. Mass Spectrom. 9, 1067 (1995).
17. D.C. Stahl et al., J. Am. Soc. Mass Spectrom. 7, 532 (1996).
18. C.L. Gatlin et al., Anal. Chem. 72, 757 (2000).
19. G. Schmelzeisen-Redeker, L. Buetfering and F.W. Roellgen, Int. J. Mass Spectrom. Ion Proc. 90, 139 (1989).
20. M. Labowsky, J.B. Fenn and J. Fernandez de la Mora, Anal. Chim. Acta 406, 105 (2000).
21. G. Wang and R.B. Cole, Anal. Chem. 66, 3702 (1994).
22. Y.P. Ho, P.C. Huang and K.H. Deng, Rapid Commun. Mass Spectrom. 17, 114 (2003).
23. A. Antonopoulos et al., Rapid Commun. Mass Spectrom. 17, 122 (2003).
24. L. Moroder et al., Biopolymers 22, 481 (1983).
25. L. Hilsted and J.H. Rehfeld, Anal. Biochem. 152, 119 (1986).

High-Speed Laser MS for Precise, Prep-Free Environmental Particle Tracking
April 21st 2025Scientists at Oak Ridge National Laboratory have demonstrated that a fast, laser-based mass spectrometry method—LA-ICP-TOF-MS—can accurately detect and identify airborne environmental particles, including toxic metal particles like ruthenium, without the need for complex sample preparation. The work offers a breakthrough in rapid, high-resolution analysis of environmental pollutants.