Scattering Impact Analysis and Correction for Leaf Biochemical Parameter Estimation Using Vis–NIR Spectroscopy
Simulated leaf spectral data were generated to analyze scattering impact and then compared to experimental data to validate the conclusions of the simulation.
In this article, the scattering impact on the estimation of leaf biochemical parameter content such as chlorophyll and water from visible–near infrared (vis–NIR) spectra was systematically evaluated. Simulated leaf spectral data involving scattering levels and interesting biochemical concentrations were generated by a leaf property model (PROSPECT) to analyze the scattering impact. Experimental data from six levels of Epipremnum aureum leaves were examined to validate the conclusion obtained from the simulated situation. To quantitatively describe the scattering impact, we defined the sensitivity function of model errors caused by scattering. Then we applied four preprocessing methods for scattering correction to eliminate the scattering effect: multiplicative scattering correction (MSC), extended multiplicative scatter correction (EMSC), optical pathlength estimation and correction (OPLEC), and orthogonal signal correction (OSC). The results show that scattering impact has a larger and more sensitive influence on water estimation than chlorophyll estimation. Simultaneously, results indicate that OPLEC is an optimal scatter correction method for chlorophyll estimation with the raising of model prediction capability by 39.04% for simulated data and 27.7% for actual data. However, OSC and OPLEC have similar correction effects for water estimation in theory.
Leaf biochemical parameters such as chlorophyll and water content can provide valuable insight into the physiological performance of plants. Traditional wet chemical analysis methods, such as extraction and high performance liquid chromatography (HPLC) techniques, require destruction of measured leaves. Hence, they are not suitable for inspecting the change of physiological state or biochemical parameters for a single leaf over time. In addition, these processes are time consuming, expensive, and impractical for making wide assessments on the health of the plant. In contrast, visible–near infrared (vis–NIR) spectroscopy analysis is a nondestructive, rapid, and applicable technique in different spatial scales. Accordingly, it is widely used in the plant parameter estimation in the level of plant canopy and leaf (1–6).
To successfully use vis–NIR spectroscopy techniques in agriculture applications, the calibration model must provide a stable, predictive capacity against perturbations from different measured objects and measurement conditions; thatis, the model must be robust. However, when taking the physical characteristics of leaves into account, a number of issues arise to prevent the requirements from being satisfied, such as differences in species, healthy states, and growing states. These differences complicate the leaf spectrum and induce additional mendacious or nonlinear factors. A total calibration model with a large number of representative samples can eliminate the influence, to a certain extent, by using multivariate soft modeling techniques such as principal component analysis (PCA), partial least squares (PLS), and least squares support vector regression (LS-SVR). Although the multivariate soft modeling techniques can more or less compensate for the effects caused by scattering light, the model robustness is sacrificed (7). From the view of the scattering mechanism, this has two effects on the spectra (8): firstly, it increases photon losses and the illusive absorption information is thus added to the absorbance spectra; secondly, the more a photon is diffused, the more absorption possibility will be obtained. Thus, the shape of the spectra is additionally modified based on the wavelength. An appropriate preprocessing method to correct the spectra must be used before building the model.
Presently, scattering correction methods are widely applied, discussed, and compared in the prediction of powder or turbid medium (9–11). But there are fewer publications about influences of scattering on plant leaf reflectance spectra. Leaf analysis is the most important tool for evaluating the nutrient and water status of plants and for guiding its fertilization and irrigation. Studies have indicated that scattering of leaves is mainly from the fluctuating state of surface and organelle distribution existing in the cells (12–15).
This article will focus on the analysis of scattering effect influence and the way to correct it effectively when the chlorophyll and water content are estimated from leaf reflectance data. To obtain a comprehensive analysis, large numbers of simulative leaf reflectance data sets with varying biochemical information by a leaf optical properties model (PROSPECT) were employed. In addition, experimental data was used to validate the conclusion. The goals of this study are to analyze the influence and sensitivity caused by light scattering in the leaf biochemical parameters estimation from vis–NIR spectroscopy and to compare four preprocessing methods to present an effective preprocessing method for correcting the light scattering effects.
Experimental
Simulated Data Acquisition
The experimental strategy adopted in this study was to obtain the leaf diffuse spectral data simultaneously, which included a wide range of chemical matter concentrations and large variability in all of other factors that influence leaf reflectance spectra. Our previous experiences in collecting samples with a large range of biochemical parameter concentrations indicated that the required time and resources were difficult to carry out. Therefore, the leaf optical properties model, PROSPECT, was used for generating spectral data to satisfy the analysis requirement.
PROSPECT is a useful radiant transfer model used to simulate leaf directional-hemispherical reflectance and transmittance over the whole optical domain (400–2500 nm) (16,17). In the model, scattering is described by the refractive index of leaf materials (n) and the parameter (N) characterizing the mesophyll structure; absorbance is modeled using pigment concentration (Cab), water depth (Cw), dry matter concentration (Cm), and the corresponding specific absorption coefficients (Kab, Kw, and Km). It is indicated when the reference spectra were obtained using the integrating sphere coated with BaSO4 attached to a PerkinElmer spectrophotometer (Waltham, Massachusetts), that the root-mean-square errors (RMSEs) of simulated reflectance spectra were less than 0.03 (17). The PROSPECT model has been used in many studies for estimation of leaf water content (18) and leaf chlorophyll content (19,20). Such studies have exploited the ability of the PROSPECT model to rapidly produce large data sets required for supporting the necessary statistical analysis, which was of particular importance in the present study.
As mentioned above, it is known that scattering information in leaf reflectance spectra is decided by N and n together. Relevant research indicates that leaf equivalent refraction index n is almost constant for different species and the differences between wavelengths are also small. Therefore, the structure parameter N only was considered in this paper. The parameter ranges for PROSPECT were derived from PROSPECT-4 (21), which provides distinction for in vivo specific absorption coefficients for each biochemical constituent and determines an average refractive index of the leaf interior. It has the advantage of the veracity of spectral simulation and the precision of model inversion.

Table I: Input variable ranges of PROSEPCT model used for scattering effect analysis
To analyze how the scattering effects influence the estimation of biochemical parameter contents from leaf diffuse spectra, seven data sets of leaf reflectance (100 samples per set) were generated by PROSPECT. The concentrations of biochemical parameters, which are the same for each data set distributed normally in the given range. N changed from 1 to 4 with the interval of 0.5 (shown in Table I). The typical spectra for different scattering levels with the same biochemical parameter concentrations (Cab = 67.94 µg/cm2 , Cw= 0.0225 cm, Cm= 0.1002 µg/cm2 ) are shown in Figure 1.

Figure 1: Typical leaf reflectance spectra with different scattering levels.
To compare the efficiency of the four scattering correction methods adopted in the estimation of leaf chlorophyll and water content, another set of representative data containing a wide range of chemical matter concentrations with random distributing of N (Table II) was generated. In addition, the number of calibration samples had to be large enough to eliminate the improvement of model prediction ability afforded by the total PLS model. In this study, 1100 reflectance spectra were simulated, and 1000 spectra were employed for building the calibration model; another 100 spectra were used for testing the model performance as the outside test data.

Table II: Input variable ranges of PROSPECT model used for scattering methods comparison
Actual Data Acquisition
Structure parameter N denotes the species and thickness of plant leaf in practice. In this study, only scattering caused by thickness differences of the same species was taken into account to avoid alternating influence. Epipremnum aureum was used as a representative plant because the thickness of different locations for the same leaf decreases gradually from leaf root to leaf apex. It is theorized that the chlorophyll and water content are distributed symmetrically for the same leaf; therefore, the spectra variations from different locations of a leaf analysis are caused by light scattering.
Samples and Spectroscopic Measurements
Six Epipremnum aureum leaves with different green and sapless levels were selected. All of them were healthy and homogeneous in color without anthocyanin pigmentation or visible symptoms of damage. Spectra of six different locations per sample were measured (shown in Figure 2a), 36 sample spectra (shown in Figure 2b) were obtained as predictor variables for two different response variables: water and chlorophyll content.
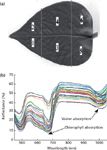
Figure 2: (a) Sample used in the experiment and (b) the spectra of leaves.
An Ocean Optics (Dunedin, Florida) spectrometer and diffuse reflectance sample accessories Y style fiber were used for spectra measurement. The light source was a white light. A white panel (Spectralon, Labsphere, North Sutton, New Hampshire) was used as a 100% reflectance standard for all measurements. The parameters of the spectrometer were as follows: spectrum scanning range, 350–1050 nm; number of pixels, 3648; integration time, 15 ms; average time, 20 ms; width of smooth window, 3.
The data were stored in the form of R (reflectance). Because of the low spectral intensity of the halogen lamp used below 450 nm and the resulting noise in the measured spectra, only reflectance data above this wavelength were considered.
Chemical Analysis
Each leaf was cut into two parts and arranged for the measurement of chlorophyll and water content, respectively. The half for chlorophyll was cut into fragments and extracted with 80% aqueous acetone solution and then centrifuged. The absorption spectra of the acetone extract was measured with the same spectrophotometer. The concentration of chlorophyll (a) and (b) was calculated based on the absorbance measured at 646.6, 663.6, and 730 nm according to the Porra formula (22). Another half was used to obtain water content by roasting. First, fresh weight (FW) was recorded using an analytical balance and leaves were dried at 105 °C in an oven for 15 min. Then the temperature was dropped down to 80 °C until the leaf weight was constant. Leaf relative water content (RWC) was calculated using the following equation:
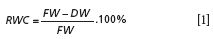
Methods
Commonly, the methods for correcting light scattering can be divided into two types. One is aimed to modify the additional spectra information caused by scattering that is usually regarded as a baseline shift of the spectra. The additional information is modeled and corrected in a more elaborate preprocessing stage. Representatives of these methods are multiplicative scattering correction (MSC) (23) and extended multiplicative scattering correction (EMSC) (8). Another method looks at the geometrical space constructed by the scattering information. Then the scattering effects are eliminated by projecting the raw spectra onto the orthogonal complement of the space, for example orthogonal signal correction (OSC) (24).
The method of optical pathlength estimation and correction (OPLEC) (25) is the combination of two ideas. It adopts the scattering model presented by Martens (as in reference 7), employs the theory of orthogonal projection, and eliminates parts of independent scattering effects with chemical matter concentration. Then, based on the assumption that there are J kinds of matter independent from each other, two established equations of linear regression are used to model the dependent scattering on the target concentration statistically. Consequently, the scattering effects are absolutely corrected without any pure spectrum information.
Multiplicative Scattering Correction
MSC is the common preprocessing technology for vis–NIR spectra. It was first introduced by Martens and colleagues in 1983 (23). Its principle is that light scattering adds an additional factor and multiplicative factor onto the ideal chemical matter absorption spectra. Thus, the measured spectra with the scattering can be expressed as the following equation:

After the estimation of the corresponding coefficients modeled by least square, the spectral correction can be expressed as

Where Xi is a measured spectrum, Xref is a reference spectrum, and ai and bi are scalar parameters obtained by calibration.
Usually the reference spectrum is the average spectrum of all the calibration samples. MSC can correct the light scattering effects caused by particle size differences when the concentration of interesting chemical matter varies only slightly, otherwise the correction will fail. MSC is based on the assumption that the scattering coefficient is dependent on wavelength. In fact, the assumption is not true. EMSC, as a development of MSC, takes account of the wavelength effects and synchronously uses a priori knowledge about the interest of chemical information.
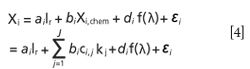
Where the function of f(λ) can be a first- or second-order polynomial or logarithmic function of wavelength. Each of them may be the best in different situations. The detailed discussion can been found in the literature of Rinnan (11). The results concluded by our team prior to this paper indicated that for leaf vis–NIR diffuse reflectance spectra, the best fitting function is the logarithmic function (26). Therefore, the form in the terms of logarithmic function was used in this study.
Optical Pathlength Estimation and Correction
OPLEC (25), proposed by Chen in 2006, resolves the problem that EMSC can't be wildly used due to a lack of the pure spectrum of chemical matter. It includes the following steps:
• Remove the additional factor and wavelength function through the projection of the measured spectrum Xi onto the orthogonal complement of the space spanned by the row vector of P = [Ir,f(λ)]
• Constant J denotes the number of kinds of matter in the sample. The projection matrix can be expressed as follows:
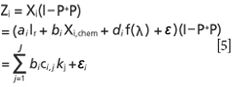
Where kj is the special chemical matter pure spectrum or some unattached influence factor after projection in the sample. bi is the pathlength of the ith sample, ci,j is the percent of the jth target concentration.
• Suppose that the first component is the interesting component, then equation [5] can be reexpressed as
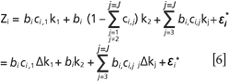
Where Δkj = kj-k2, Zi simultaneously has the linear relationship with bici1 and bi.
• Calculate the coefficient through a series of mathematic transformations.
• Construct two calibration models using a multivariate linear calibration method such as PLS, where Ic is a column vector with its elements equal to unity.
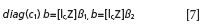
• Correct the light scattering effect of an unknown sample and predict the target concentration at the same time.
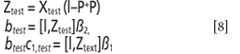
Respectively, the corrected spectrum and the target concentration will be calculated as follows:
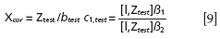
Orthogonal Signal Correction
OSC was initially proposed by Wold (24) as an adaptation of the PLS-NIPALS regression algorithm. Subsequently, it was used as a preprocessing method that focused on correcting the initial spectrum on the basis of the target concentrations. This treatment has been used to correct systematic spectral alterations such as baseline changes and multiplicative effects, which are absolutely independent of concentration. The detailed implementation of OSC was described in several references (24).
Selecting the number of OSC factors and internal latent variables (those involved in the removal of orthogonal information) are pivotal for the algorithm. Up to this point, the different papers published showed a lack of unified criteria about the procedure in the selection of these factors. Usually, one or two components were suggested to be removed.
Software
The PROSPECT-4 code in Matlab is available at http://teledetection.ipgp.jussieu.fr/prosail/. The scattering correction algorithms and all the calculations were implemented in Matlab 7.10 (Mathworks, Inc., Natick, Massachusetts) .The calibration process was conducted using the statistical software of Unscramble 7.6 (CAMO Software AS, Oslo, Norway).
Result and Discussion
Scattering Influence and Sensitivity Analysis on Simulated Data
Analysis of scattering influence and sensitivity were conducted in this section. PLS regression models were built for different scattering levels and the root-mean-square error of prediction (RMSEP) denoted the precision of biochemical parameters estimation. Then a small disturbance of ΔN = 0.1 was added to each level of N; the function of sensitivity is defined as follows:
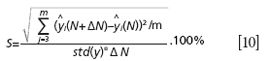
Where ŷi (N) is PLS model predictive value of the ith sample with the scattering level of N, m is the sample number, std(y) is the variance of the target concentration. S denotes the relative model error caused by scattering perturbation at different scattering levels.
From the PROSPECT model, it is known that leaf mesophyll structure parameter N is associated with the cellular arrangement. For leaves from plants of different species or different senescence phases of homogeneous plants, parameter N is different. Moreover, it is noticed that N cannot be measured directly by any physical or chemical method. In relevant research, two empirical methods based on the correlation of N and specific leaf area (SLA: leaf area per unit leaf dry weight) are usually adopted. One of which is given by Jacquemoud and Baret (14), who show that
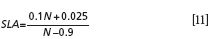
Another mentioned by Ceccato (6) is defined as

The relationship of N and SLA was shown in Figure 3. SLA denotes the leaf area unit leaf dry weight, which can be simply regarded as the degree of leaf thickness and compactness. It is clear that N increases with the decrease of SLA, for example photon losing caused by light scattering will be reduced when the cellular arrangement is compact. In addition, it can be found that N in the 1–2 range leads to an abrupt change of SLA.
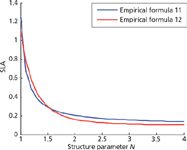
Figure 3: The relationship between N and SLA.
Results of PLS models performed on each spectra matrix and biochemical parameters are shown in Table III. The conclusion is that scattering effects are inverse proportion to structure parameter N. In addition, it is clear that scattering effects mainly contribute to the first component. The explained ability of first component of spectra matrix descends with the decrease of scattering levels. A total model containing all the scattering levels was established. The RMSEP decreased from 1.69 to 1.47 (13.02%) for chlorophyll content estimation and from 1.3 to 1.2 (7.69%) for water content estimation. The indication is that the improvement of model precision derived from the total model is small. Therefore, it can be concluded that the scattering effects have a larger influence on the water content estimation. From the view of sensitivity, it is clearly found that though S of both water and chlorophyll decreases followed by increases of N, scattering is more sensitive for water estimation than chlorophyll (for instance, when N = 1, S =1 6.24 for water and S = 8.34 for chlorophyll).
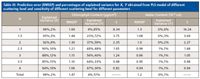
Table III: Prediction error (RMSEP) and percentages of explained variance for X, Y obtained from PLS model of different scattering level and sensitivity of different scattering level for different parameters
Scattering Correction on Simulated Data
The predicted ability of PLS models obtained after preprocessing by four methods is investigated in this section. All discussions are based on the PLS regression model. To ensure a robust PLS model, 1000 calibration samples were split into 20 consecutive cross-validation segments with each of the segment containing 50 samples. The results are shown in Table IV. RMSECV denotes the precision of the calibration model. RMSEP and |e|max denotes the predictive accuracy of the model. PCA analysis was conducted for the original and all corrected spectra matrix. Figure 4 shows the original spectra after mean center (Figure 4a) and the corrected spectra by the preprocessing methods after mean center (Figures 4b–4g). The upper part of each figure is the relativity between score of first principal component with the scattering level. Capital R denotes the correlative coefficient. From the figures, it can be found that the absorption peak of chlorophyll at 680 nm becomes prominent for all the spectra after preprocessing. At the same time, R from each preprocessing method is lower than the original data. Therefore, all the methods are effective to correct the scatter effects.

Table IV: Comparison criteria for different preprocessing methods
Table IV shows that the best preprocessing method of scattering correction is OPLEC for the estimation of chlorophyll concentration. Compared with the results from original data (shown in Figure 4a), the RMSEP decreases from 1.87 to 1.14, the prediction capability rises by 39.04% and simultaneously, |e|max reduces from 8.1 to 3.71. The accuracy of the model is improved by 53.83%. In addition, R decreases from 0.778 to 0.63, which is close to the results from EMSC and MSC (shown in Figures 4b and 4c). The spectra preprocessing by OPLEC and the scatter spot figure of R are shown in Figure 4d. It is found that spectra corrected by OPLEC have only one, instead of two , wave envelopes around 680 nm, which is different from MSC and EMSC. This is in accords with the absorption characteristic of chlorophyll that is consistent with result corrected by OSC (shown in Figure 4f).

Figure 4: The spectra of calibration samples after mean-centering preprocessing by different methods and the relativity between score of first principal component with the scattering level. (a)â(g) respectively, corresponding with original, preprocessing by MSC,EMSC,OPLEC, and OSC; (d) and (f) are for chlorophyll, (e) and (g) are for water.
As far as the methods of MSC and EMSC are concerned, scattering effects are assumed as shifts in baseline, and the average spectrum is used as a reference spectrum to eliminate the shift. When the range of concentration of an interesting target is large, the spectra of a sample with larger or smaller concentrations are over-corrected. The actual chemical absorption information is corrected as a baseline shift. Thus, the false information around 700 nm appears and the corrected effect is not perfect; although, there are some improvements in the aspect of model precision compared with the original data.
Considering the effect corrected by OSC, though R is 0.354, which is smaller than OPLEC, the precision and predictive capacity of calibration models are not perfect. This will be explained below.
For the estimation of the water content from leaf spectra, the best result was obtained by OSC treatment. RMSEP is 0.46 using a model of k = 2 and LVs = 14 with the |e|max = 1.65. The predictive accuracy of model was raised by 63.17% and the prediction capability raised by 58.18%. Besides, the calibration model derived from OPLEC (shown in Figure 4e) achieves similar result with the RMSEP = 0.47 and |e|max = 1.85 using a model of J = 6 and LVs = 6.
Figure 5 shows the best value of J for chlorophyll and water estimation from OPLEC. The meaning of J is not the value of actual matter but of unattached signal. The best J results for chlorophyll can be found in Figure 5a, where J = 2. This is different from the value obtained for water, where J = 6, shown in Figure 5b. Compared with water, chlorophyll is the main compound dominating the optical character in the vis–NIR region. It has an obvious absorption peak and can easily be extracted from spectra with J = 2. But the situation of water is different. The absorption peak of water in the shortwave NIR range (around 970 nm) is weak. Thus, the signal is weak even though a great deal of water exists in the leaf. It is difficult to extract weak information from the complex spectra. The value of J for water estimation (J = 6) is bigger than chlorophyll.

Figure 5: RMSECV versus the number of Zi,base used in preprocessing by OPLEC method for simulated data. (a) chlorophyll, (b) water.
Results from MSC and EMSC indicate that they have the same scattering correction capacity for water estimation. Compared with the results from the original model, the predictive capability of the MSC model increased by 36.4%, larger than 21.4% for chlorophyll.
The success of OPLEC is attributed to its avoiding the request of matter pure spectrum, which limits the use of EMSC. The coefficient b has improved accuracy by introducing the concept of the number of substantial. Two PLS models ascertained the best J were used to fit the accurate coefficient b. The scattering effects independent and dependent of concentration are simultaneously removed. Consequently, the corrected spectrum is closer to the ideal matter absorption spectrum, and variations of chemical matter in the spectrum are more prominent. Because the scattering model used in OPLEC is the same with EMSC, the result of PCA from spectra corrected by OPLEC is close to EMSC.
Scattering effects removed by OSC are unrelated to the concentration of interesting parameters. Whereas, not only scattering effects but also other information unrelated to the concentration of target matter are removed by OSC preprocessing. This is the reason why the correlative coefficient R was lower than OPLEC. For a plant leaf, the scattering effects are caused by cell physical structure and the organelle distribution. The chlorophyll exists mostly in the chloroplast. Hence, the distribution density of chloroplast indeed impacts the scattering effects. However, this is not a problem for water. Water in the leaf mainly consists in the vacuole, which is regarded as a translucent solution and doesn't induce the scattering. Therefore, the performance of OSC is not good for chlorophyll, but similar to OPLEC for water. The scatter spot figure of R and the spectra corrected by OSC for water are shown in Figure 4g. The results revealed that the relativity of score of 1th PC and N for water was less than chlorophyll. It also proved that scattering has more influence on water estimation than chlorophyll.
Scattering Correction on Actual Data
In this section, all of the preprocessing methods applied to a quantitative spectroscopic task involving 36 leave samples is discussed. Some general performance differences are revealed (shown in Table V). RMSECV is obtained from the leave-one-out cross-validation.

Table V: Comparison criteria for different preprocessing methods on actual data
As a first observation, the actual leaf diffuse spectra are more complicated. More interferential information makes it difficult to extract chemical information from leaf spectra, but nearly all the preprocessing methods are effective to improve the precision of the calibration model.
In the case of the chlorophyll models, scattering correction of MSC, EMSC, and OSC were unable to extract more detailed information than the PLS model without pretreatment (all with 3 LVs) though the precision of the model improved slightly. It is denoted that the main influence factor is not removed. Albeit complex, the accuracy of model from OPLEC with J = 18 and LVs = 14 (shown in Figure 6a) is improved by 53.32%. This result is close to the simulated data results.
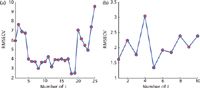
Figure 6: RMSECV versus the number of Zi,base used in preprocessing by OPLEC method for actual data. (a) chlorophyll, (b) water.
In the case of water models, because of the scattering effects irrelevant to the water concentration, the information from spectra corrected by MSC and EMSC can be extracted by PLS in more detail. The LVs number of the PLS model increased to LVs = 10 and LVs = 9 with RMSECV = 1.41 and 1.46. Moreover, with regard to the differences from simulated data, the results obtained from OPLEC are not as good as those from OSC with RMSECV = 0.67 and |e|max = 3.08. The best J of OPLEC is 5 (shown in Figure 6b), which is not in accord with the theory. The number of samples is small and the colinearity between samples is strong; therefore, J was calculated only from 1 to 10.
For both chlorophyll and water estimation, EMSC models were not as good as MSC. Presumably, the differences were the result of the small range of concentration of the biochemical parameters, and sequentially scattered effects induced by the dependent wavelength are not distinct. Figures 7a and 7b show the RMSECV attained by all preprocessing methods for chlorophyll and water. It is seen that OSC tends to over-fit the results, but this can be avoided by carefully selecting the number of OSC factors and internal latent variables in practice.

Figure 7: Predictive performance of PLS models built on the leaf spectra preprocessing by different methods. (a) chlorophyll content, (b) water content.
Figures 8a and 8b show the scatter plots of predicted concentration versus the true concentration of chlorophyll and water, respectively. It can be seen that compared with the result of original data without scattering correction, the six samples yield both chlorophyll and water concentration predictions that are close to the theoretical expectation (blue line) after appropriate preprocessing. Hence, the scattering effects evident in the original data have been eliminated.

Figure 8: The scatter plots of predicted concentration vs. true concentration of chlorophyll and water content. (a) chlorophyll. (b) water.
Conclusions
In this study, the simulative leaf spectra with variations from different scattering levels and the actual leaf scattering spectra from identical species were used to assess the scattering impact and sensitivity on estimation of biochemical parameters content (chlorophyll and water). Preprocessing methods for correcting scattering effects were applied to preprocess the spectra. A PLS regression method constructing the calibration model was used for analyzing the scattering impact and determining the efficiency of the preprocessing method. The conclusions are as follows:
- Scattering is in inverse proportion to leaf structure parameter N. At the same time, the scattering effects are not the same for different parameter estimations. Because of the physiological properties of the leaf, the estimation of water concentration is easily affected by scattering. In addition, the optimal preprocessing method for different parameters is not same.
- For the estimation of chlorophyll concentration from the diffuse reflectance, the best preprocessing method is OPLEC, which can raise the model prediction capability by 39.04% and 27.7% for simulated data and actual data. This conclusion is expected because it eliminates the scattering effects independently and dependently with analyte concentration simultaneously.
- For the estimation of water concentration from the diffuse reflectance, theoretically, OPLEC and OSC achieve similar performances with respect to the accuracy of the predictions. This is because the scattering impacts on the leaf spectra are independent with water concentration. But under the realistic measurement conditions, in which the number of calibration sample is not large enough, the performance of OPLEC will not be as good as OSC.
Acknowledgments
This study is supported by National Natural Science Foundation (Nos.60708026), Beijing Municipal Training Program Foundation for the Excellent Talents (20081D1600600348) and Programs for Changjiang Scholars and Innovative Research Team in University (PCSIRT) of China (IRT0705).
References
(1) A.A. Gitelson, Y. Gritz, and M.N. Merzlyak, J. Plant Physiol. 60, 271–282 (2003).
(2) R. Colombo, M. Meroni, and A. Marchesi, Remote Sens. Environ. 112, 1820–1834 (2008).
(3) A.B. George and J.G. Ferwerda, Remote Sens. Environ. 112, 1614–1632 (2008).
(4) D.A. Sims and J.A. Gamon, Remote Sens. Environ. 81, 337–354 (2002).
(5) L.H. Xue and L.Z. Yang, ISPRS J Photogram 64, 97–106 (2009).
(6) P. Ceccato, Remote Sens. Environ. 77, 22–33 (2001).
(7) H.Martens, J.P. Nielsen, and S.B. Engelsen, Anal. Chem. 75, 394–404 (2003).
(8) J.M. Roger, F. Chauchard, and V. Bellon-Maurel, Chemometr. Intell. Lab. Syst. 66, 191–204 (2003).
(9) S. Preys, J.M. Roger, and J.C. Boulet, Chemometr. Intell. Lab. Syst. 91, 28–33 (2008).
(10) S.N. Thennadi and E.B. Martin, J. Chemometrics. 19, 77–89 (2005).
(11) å. Rinnan, F.V. Berg, and S.B. Engenlesn, Trends Analyt. Chem. 28, 1201–1222 (2009).
(12) L. Fukshansky, A.M. Remisowsky, and J. McClendon, Photochem. Photobiol. 57, 538–555 (1993).
(13) J.T. Woolley, Plant Physiol. 47, 656–662 (1971).
(14) M.R. Slaton, E.R. Hunt, and W.K. Smith, Am. J. Bot. 88, 278–284 (2001).
(15) D.A. Reicosky and J.W. Hanover, Plant Physiol. 62, 101–104 (1978).
(16) S. Jacquemoud and F. Baret, Remote Sens. Environ. 34, 75–91 (1990).
(17) S. Jacquemoud, S.L. Ustin, and J. Verdebout, Remote Sens. Environ. 56, 194–202 (1996).
(18) P. Ceccato, S. Flasse, and S. Tarantola, Remote Sens. Environ. 77, 22–33 (2001).
(19) N.C. Coops and C. Stone, Aust. J. Bot. 53, 417–429 (2005).
(20) G. Le Maire, C. Francois, and E. Dufrene, Remote Sens. Environ. 89, 1–28 (2004).
(21) J-B. Feret, C. Francois, and G.P. Asner, Remote Sens. Environ. 112, 3030–3043 (2008).
(22) R.J. Porra, W.A. Thomson, and P.E. Kriedmann, Biochim Biophys Acta. 975, 384–394 (1989).
(23) H. Martens, S.A. Jensen, and P. Geladi, Multivariate Linearity Transformations for Near Infrared Reflectance Spectroscopy, O.H.J. Christie, Ed. (Norway, 1983) pp. 205–234.
(24) S. Wold, H. Antti, and F. Lindgren, Intell. Lab. Syst. 44, 175–185 (1998).
(25) Z.P. Chen, J. Morris, and E. Matrin, Anal.Chem. 78, 7674–7681 (2006).
(26) Q.X. Zhang, G.J. Zhang, and Q.B. Li, Spectros. and Spec. Anal. 30, 1310–1314 (2010).
Qianxuan Zhang, Qingbo Li, and Guangjun Zhang are with the Department of Instrument Science and Opto-electronics Engineering at BeiHang University in BeiJing, China.

NIR Spectroscopy Explored as Sustainable Approach to Detecting Bovine Mastitis
April 23rd 2025A new study published in Applied Food Research demonstrates that near-infrared spectroscopy (NIRS) can effectively detect subclinical bovine mastitis in milk, offering a fast, non-invasive method to guide targeted antibiotic treatment and support sustainable dairy practices.
Karl Norris: A Pioneer in Optical Measurements and Near-Infrared Spectroscopy, Part II
April 21st 2025In this two-part "Icons of Spectroscopy" column, executive editor Jerome Workman Jr. details how Karl H. Norris has impacted the analysis of food, agricultural products, and pharmaceuticals over six decades. His pioneering work in optical analysis methods including his development and refinement of near-infrared spectroscopy, has transformed analysis technology. In this Part II article of a two-part series, we summarize Norris’ foundational publications in NIR, his patents, achievements, and legacy.
New Study Reveals Insights into Phenol’s Behavior in Ice
April 16th 2025A new study published in Spectrochimica Acta Part A by Dominik Heger and colleagues at Masaryk University reveals that phenol's photophysical properties change significantly when frozen, potentially enabling its breakdown by sunlight in icy environments.