Six Key Errors to Avoid for Routine Analysis Using Inductively Coupled Plasma-Mass Spectrometry (ICP-MS)
Special Issues
Inductively coupled plasma-mass spectrometry (ICP-MS) is a key technology for the detection and quantitation of trace elements in a wide variety of sample types, mainly because of its high detection power, dynamic range, and versatility. However, in order to leverage the full potential of the technology, a few key errors should be avoided.
Pay Attention to Acid and Vial Purity!
ICP-MS is a technology for the analysis of elemental impurities at trace and even ultratrace levels. This implies, however, that backgrounds for the analytes of interest must be as low as possible. Otherwise, false positive results and poor detection limits may cause significant follow-on work in a routine operating laboratory. For many elements, there is likely no background contamination found in dilution water, acids, and the vials used during sample preparation. For other elements, especially alkali earth and transition metals, there may be significant amounts leaching out from plasticware from the vials and caps. Note that when changing from batch to batch or between different brands, at least a preliminary test for the purity of vials is highly recommended.
The purity of acids also may add to the background signal observed during an analysis. Acids and other reagents typically used in elemental analysis, such as nitric and hydrochloric acid or hydrogen peroxide, are available in different purities, but for trace and ultratrace analysis, only the highest purity should be used.
As an alternative, acids of less purity can be purified using sub-boiling distillation. This is a cost-effective way to increase the purity of a less expensive acid.
Consider the Sample Matrix Composition
Although ICP-MS is known to be highly tolerant to different sample matrices, there are some limitations. In general, the level of dissolved solids should not exceed approximately 0.3 to 0.5%, unless the sample introduction system is configured with, for example, a gas dilution unit. High amounts of total dissolved solids typically lead to signal suppression, which can be corrected for when using an appropriate internal standard. Since the internal standard is affected in a similar way, the observed intensities can be corrected for; however, most regulatory guidelines give limits for internal standard suppression, and these limits need to be applied (typically 80–120%).
It is not always the presence of salts or other solids that can negatively impact results. In some cases, the composition of the matrix itself may lead to adverse effects. For example, the presence of organic compounds may influence the viscosity and change the efficiency of the nebulizer. If carbon is present in a sample (for example, when sugar-containing samples are simply diluted before analysis), there is a positive bias for the observed signal intensity of arsenic and selenium. The presence of carbon may lead to an increase in signal intensity of up to 10 times, leading to grossly inaccurate results. This effect is not observed in samples that were previously digested; for example, using a microwave assisted digestion procedure including hydrogen peroxide. Here, all carbon-containing compounds are destroyed, and evaporate as expended CO2 from the sample.
Maintenance Is Performed Too Often
Although ICP-MS is a technique for trace and ultratrace analysis, and cleanliness of vials and reagents is a key to achieving lowest detection limits, daily routine maintenance of the sample introduction system components is often not required. In fact, long-term stability and performance of ICP-MS systems is often achieved the opposite way.
The reason for this is the formation of an equilibrium state in the interface region (the sample and skimmer cones transferring ions from atmospheric pressure into high vacuum). Due to the nearby plasma, the cones are running at elevated temperatures when the system is in operation. There is a constant deposition and evaporation of material from the cones, which initially leads to the observation of signal drift; however, this process contributes to the robustness of the system signal in the long run. Performing routine maintenance, such as cleaning of the cones, will destroy the equilibrium conditions and lead to elevated system drift once again.
Determining when cones need to be cleaned or replaced is often not easy. It can be helpful to monitor, for example, signal-to-background for certain analyte and interference combinations. For example, monitoring the ratio between 59Co and m/z 51 (35Cl16O+) using a solution containing 1 ppb Co in 0.5% HCl can be a powerful indicator. For a clean system, a ratio of greater than 30:50 can easily be achieved, typically corresponding to a reduction of 35Cl16O+ of several orders of magnitude. Through constant use of the system, potential contamination (for example, 12C39K+) causes the observed ratio to decrease with time. This can be partially compensated for by adjusting the collision-reaction cell (CRC) gas flow, leading to an increase of the interference removal capacity in the cell. Over time, the increase of the gas flow will negatively impact sensitivity, so that at some point, neither the typical 59Co+/35Cl16O+ ratio nor the minimum sensitivity can be achieved. At this point, cone maintenance is strongly recommended.
Use the Right Collision–Reaction Gas for Interference Removal
The occurrence of interferences, in particular polyatomic interferences formed from all elements present in a sample, has been a major challenge when working with ICP-MS. Today, almost all instruments are equipped with collision-reaction cell systems (CRCs) to eliminate these interferences. Mostly, kinetic energy discrimination (KED) is the way to remove polyatomic interferences, using an inert gas such as helium. In the most affected region of the mass spectrum, between m/z 40 and roughly 100, the elimination of polyatomics using KED is highly efficient and allows the removal of even highly abundant interferences (such as 40Ar35Cl+ interfering with 75As+). Intelligent design of the cell can also allow to run elements with lower (lithium or magnesium), and higher mass (mercury, thallium, lead) under the same conditions without sacrificing detection limits. Running an analysis in one single mode can significantly improve the throughput of a laboratory since gas switching can be avoided. This can save around 10–20 s of analysis time per sample.
In some cases, hydrogen can be an alternative to effectively suppress interferences such as 40Ar40Ar+ that effectively masks the most abundant isotope of selenium, 80Se+. Removal of the argon dimer with hydrogen allows selenium to be analyzed with the best possible sensitivity and detection limits.
While the use of reactive gases may be effective for some analytes, they may induce the formation of new and unexpected interferences, again causing significant false positive results. Only dedicated triple-quadrupole systems can overcome this limitation, as they use an additional quadrupole mass filter situated axially in front of the CRC to filter out unwanted ions before they can react and form unwanted byproducts.
Effectively Use the Linear Dynamic Range of Your Instrument
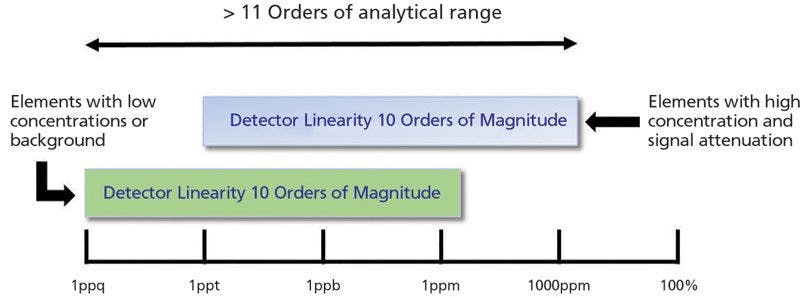
ICP-MS is known to be an analytical technique that offers a very high linear dynamic range, which allows elements to be analyzed from very low to very high concentrations (a few ng/L to a few hundred mg/L). Most instruments offer a dynamic range of 10 orders of magnitude, equivalent to the detection of count-rates as low as 0.5 cps up to almost 10 Gcps. However, this is not necessarily the analytical range, which is the concentration range for different elements present in an unknown sample. The analytical dynamic range can be significantly extended by attenuating the signal for major elements like sodium or calcium. There are options that enable the reduction of the signal intensity, such as adjustable resolution settings on the analyzing quadrupole, thus extending the dynamic range. Figure 1 shows how the typical linear dynamic range of an ICP-MS instrument can be fully leveraged to span an even larger measurement range for different elements, for example, both traces and major elements in the same analysis of a particular sample.
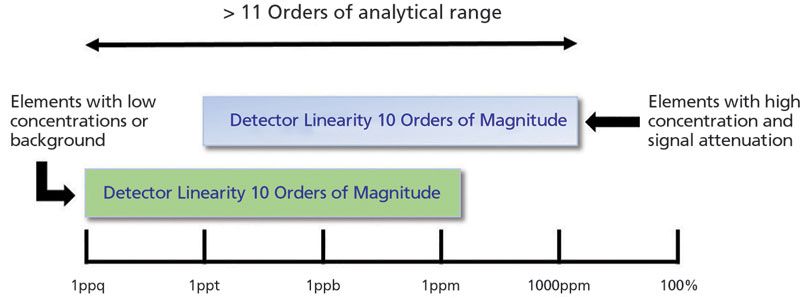
Figure 1: Detector linear range versus measurement range. Linear dynamic range of the detector versus the measurement range for different elements using ICP-MS.
Discard Uncommon Interferences Early (Doubly Charged and Isobaric)
As mentioned previously, in most cases polyatomic interferences are responsible for bias in ICP-MS analysis. However, there are other types of interferences that may critically affect results and are not straightforward to discover and resolve:
- Isobaric interferences can occur when different elements share isotopes with identical mass, such as 114Cd and 114Sn. For all elements, with the exception of indium, there is always one isotope that can be detected free from isobaric overlap. If not, isobaric interferences can be corrected mathematically if they are recognized.
- Doubly charged interferences can occur especially due to elements that have a low second ionization potential, allowing the creation of [M]++ ions. Because mass spectrometers commonly separate ions based on their mass to charge ratios (m/z), and not directly mass, these interferences affect analytes with half the mass. For example, 69Ga or 103Rh can be affected by the presence of even moderate amounts of barium (138Ba++) or lead (206Pb++), respectively.
This type of interference is even more pronounced when working with kinetic energy discrimination (KED) for the removal of polyatomic interferences!Often, the aforementioned analytes are used as internal standards, therefore the interference may not be readily discovered as a bias, but could instead be interpreted as a system drift or random spikes in the internal standard.
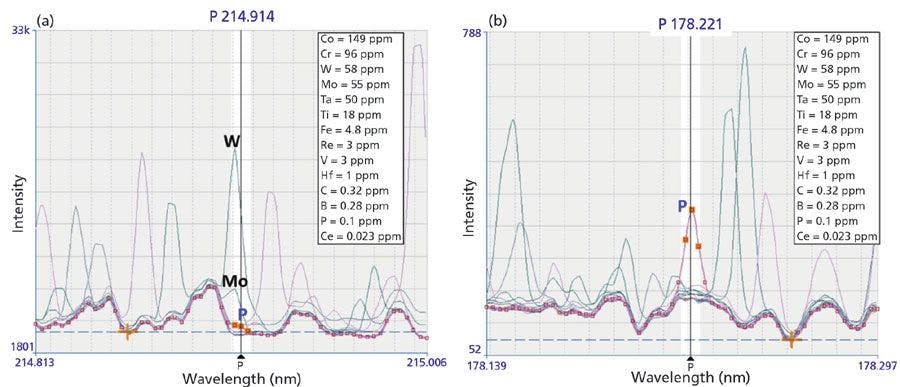
Both types of interferences can be discovered when examining full mass spectra, unveiling the presence of all elements in the sample. Figure 2 shows an example for the presence of doubly charged ions of barium. The corresponding doubly charged signals appear at half the mass of barium isotopes but can be identified visually by the typical isotope pattern. This pattern seems to appear unresolved and distorted since the mass difference between individual isotopes is now reduced to 0.5 amu.

Figure 2: Full mass spectrum showing the presence of barium and its doubly charged ions as potential interferences. Note that (a) Ba isotopes (from m/z 130 to 138) can create M++ interferences on (b) m/z 65-69, leading to interferences on 66Zn+ and 68Zn+, or 69Ga+.
Daniel Kutscher is a Product Marketing Specialist with Thermo Fisher Scientific, in Bremen, Germany. Direct correspondence to: daniel.kutscher@thermofisher.com

How Do We Improve Elemental Impurity Analysis in Pharmaceutical Quality Control?
May 16th 2025In this final part of our conversation with Harrington and Seibert, they discuss the main challenges that they encountered in their study and how we can improve elemental impurity analysis in pharmaceutical quality control.
High-Speed Laser MS for Precise, Prep-Free Environmental Particle Tracking
April 21st 2025Scientists at Oak Ridge National Laboratory have demonstrated that a fast, laser-based mass spectrometry method—LA-ICP-TOF-MS—can accurately detect and identify airborne environmental particles, including toxic metal particles like ruthenium, without the need for complex sample preparation. The work offers a breakthrough in rapid, high-resolution analysis of environmental pollutants.
FT-IR Microscopy, Part 2: Mid-IR Sampling with DRIFTS, IRRAS, and ATR
February 14th 2025Fourier transform infrared (FT-IR) microscopy using reflection methods (diffuse reflection, reflection/reflection-absorption, or attenuated total reflectance) typically requires less sample preparation than transmission. However, optimal results will depend upon the sample and, in particular, the sample surface.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)