Analysis of Unlabeled Sibutramine in Dietary Supplements Using Surface-Enhanced Raman Spectroscopy (SERS) with Handheld Devices
Counterfeit pharmaceuticals are fake or falsified drug products that pose a major threat to public health and the global supply chain. Counterfeit drugs may be manufactured using incorrect or harmful ingredients, have insufficient amount of the active pharmaceutical ingredients (APIs), or may be packaged and labeled to look like approved authentic products. Most of these drugs enter the U.S. supply chain via international mail facilities (IMFs) or express courier hubs (ECHs), and reports indicate that a substantial amount of these drug products are not Food and Drug Administration (FDA) compliant (1).
To limit the number of dangerous pharmaceutical products reaching the market, the U.S. Congress passed legislation granting FDA destruction authority for “articles of concern,” including violative products containing opioids or other nonapproved FDA drugs encountered at IMFs and ECHs (2). Laboratory analysis of these “articles of concern” for the presence of APIs and other drug substances may be needed for the FDA to exercise its administrative destruction authority. The package documentation, transit costs to FDA regulatory laboratories, analysis time, and report writing required by this process are time consuming for any significant impact of the destruction authority to be realized.
The outcome of several mail blitz operations carried out at multiple IMF facilities throughout the United States revealed the presence of suspect counterfeit low-dosage pharmaceutical products and dietary supplements containing APIs. The suspect products were found to contain labeled and unlabeled APIs using established laboratory-based methods such as Fourier transform infrared (FT-IR) spectroscopy, gas chromatography with mass spectrometric detection (GC–MS), and liquid chromatography with mass spectrometric detection (LC–MS). To reduce the need to send IMF samples to a laboratory for analysis, the FDA’s Forensic Chemistry Center has been tasked with evaluating and implementing handheld or portable analytical instrumentation to screen the contents of suspect packages for APIs in illicit medications, foreign unapproved pharmaceutical products, and unknowns (3). Rapid field-friendly methods using handheld or portable analytical tools, such as Raman spectrometers, FT-IR spectrometers, and direct analysis in real-time mass spectrometers (DART-MS) are needed to detect these APIs.
Although handheld Raman spectrometers are excellent for detecting high- concentration APIs in suspect products, sensitivity limitations often preclude their use for detecting APIs at low concentrations. This limitation can be overcome by utilizing the enhancement effect of noble metal surfaces for Raman studies. This surface enhancement effect was first documented by Fleischmann and associates, and later verified by Van Duyne, where the Raman scattering signal intensity of pyridine molecules adsorbed on the surface of roughed silver electrodes was increased by 106 (4,5). The enhancement factor (EF) achieved using silver or gold colloids can facilitate the development of an efficient analytical tool for detecting low-concentration analytes. Using handheld Raman spectrometers with an on-board matching algorithm, surface enhanced Raman spectroscopy (SERS) methods have been developed and successfully validated to identify various APIs in our laboratory. These methods have been employed for use in the field to detect opioids, phosphodiesterase type-5 (PDE-5) inhibitors, local anesthetics, appetite suppressants, alkaloids, and several other drug products (6–9). Field-deployable handheld Raman devices can provide the ability to detect suspect counterfeit drug products (10) and FDA-regulated products adulterated with undeclared APIs. As a representative example, the study presented here describes a SERS method for detection of sibutramine (Figure 1) found in adulterated dietary supplements. The SERS reference spectra of other adulterants previously observed in dietary supplements are also shown in Figure 1 (11). The development of a SERS method and implementation of a simple micro-extraction technique for sibutramine in capsules labeled as dietary supplements will be discussed.
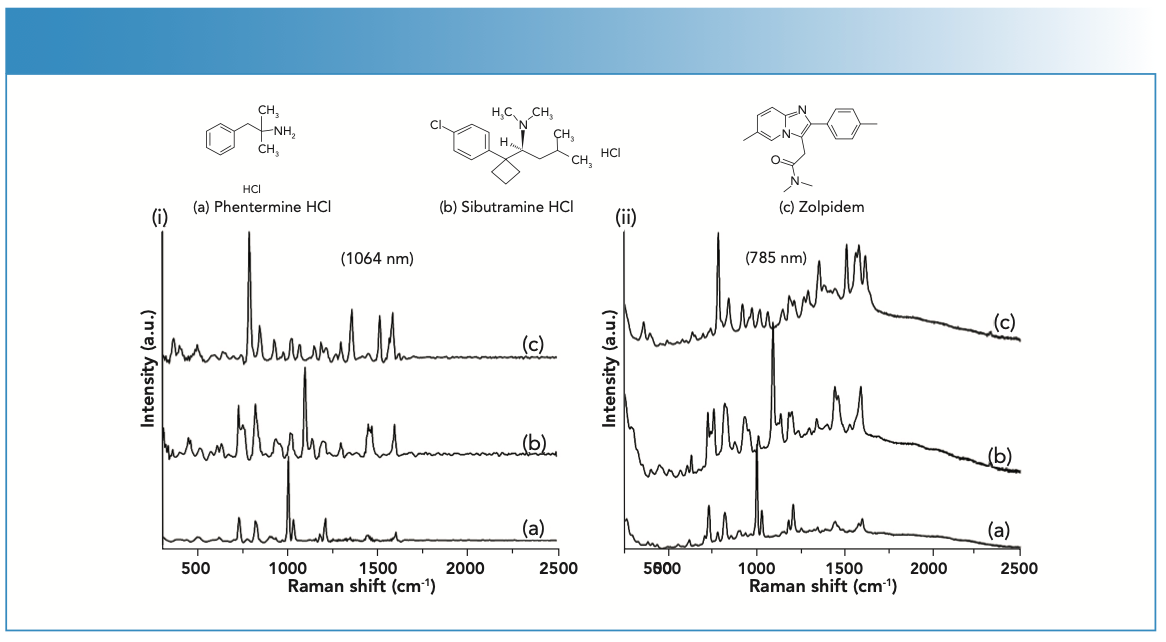
FIGURE 1: SERS spectra of (a) phentermine HCl, (b) sibutramine HCl, and (c) zolpidem. The SERS spectra were collected using a (i) Progeny ResQ equipped with a 1064 nm laser and (ii) TruScan RM equipped with a 785 nm laser. Each analyte concentration was 100 μg/mL in 10% methanol in water with colloidal silver and potassium bromide.
Materials and Methods
Sibutramine HCl, phentermine HCl and zolpidem reference standards were acquired from Thermo Scientific. Certificates of analysis for the standards were received from the vendor, which verified conformity and purity (typically >95%). Other reagents and materials used included potassium bromide, methanol (Sigma Aldrich), silver colloids (Real-Time Analyzers), 3-mL luer-lock syringes (Becton and Dickinson), Target2 PTFE 0.45-μm pore size filters (Thermo Scientific). The following equipment was used: Gilson pipetman electronic pipette (Gilson Inc.), Vortex-2-Genie (Scientific Industries Inc.), and Mettler Toledo XS205 Semi-Micro balance (Mettler Toledo).
Instrumentation
Two handheld Raman spectrometers were used: a TruScan RM (785 nm laser) from Thermo Scientific and a Progeny ResQ (1064 nm laser) from Rigaku Americas.
Samples
Dietary samples taken from adjudicated cases and previously found to contain sibutramine using GC–MS or LC–MS were used for method development purposes.
Method Development
Standards Preparation
The API standards were weighed using a calibrated analytical balance, and a stock solution of 1 mg/mL of each standard was prepared in 10% methanol in H2O and diluted to concentrations of 500, 200, 100, 75, 50, 25, 10, 5, and 1 μg/mL and 750, 500, 250, and 100 ng/mL.
A SERS signature spectrum of each API was initially collected without the addition of an aggregating agent. Next, 250 μL of silver colloidal solution was transferred to a 4 mL glass vial, followed by 250 μL of the standard (100 μg/mL) in 10% methanol in H2O and 5 μL of 3 M potassium bromide aqueous solution. The glass vial was capped and vortexed for 30 s. The sample was left to react and equilibrate for 30 s prior to conducting Raman analysis. The suitability of the SERS signature spectrum was based on visual analysis of the signal intensity obtained for each standard.
Sample Preparation: Micro-Liquid-Solid Extraction (micro-LSE)
For product that contained sibutramine, 20 mg of the capsule contents were weighed, transferred into a glass vial (a) and used for analysis. Using a pipette, 750 μL of 10% methanol in water (H2O) was added to vial (a), and the suspension was mixed on a vortex mixer for approximately 30 s to extract the analyte into the solvent. The mixture was filtered into a new clean glass vial (b) using a 0.45-μm nylon filter to remove any particulate material. The contents in vial (b) were mixed with 500 μL of silver colloid solution and the solution was vortexed for 30 s. Finally, 5 μL potassium bromide solution was transferred to vial (b) followed by vortexing for an additional 30 s. The sample was left to react and equilibrate for 30 s prior to conducting Raman analysis.
Results and Discussion
Representative SERS reference spectra of the three adulterants previously observed in dietary supplements are shown in Figure 1. The SERS spectra of sibutramine, phentermine, and zolpidem reference standards at concentrations of 100 μg/ mL were collected using handheld Raman devices equipped with either 785 or 1064 nm lasers. The SERS reference spectra collected had suitable signal-to-noise ratios for use as signature spectra, and can be discriminated from each other based on their spectral features.
Dietary supplements found to contain sibutramine using LC–MS and GC–MS were analyzed. As an example, the normal Raman and SERS spectra obtained from the analysis of a suspect capsule contents are presented in Figures 2i[a–d] and 2ii[a–d]. The normal Raman spectra of the capsule contents collected using Progeny ResQ and TruScan RM (Figures 2i[a] and 2ii [a]) did not exhibit any discernable peaks. The fluorescence observed in Figure 2ii (a) is because of the 785 nm excitation laser for TruScan RM versus 1064 nm for Progeny ResQ. The 10% mathanol in H2O extract solution spectra (Figures 2i[b] and 2ii[b]) are dominated by methanol peaks; none of these spectra exhibit peaks associated with a known adulterant. When the 10% methanol in H2O extract solution, silver colloids, and 5 μL potassium bromide aqueous solution are mixed, the Raman spectra obtained (Figures 2i[c] and 2ii[c]) depict peaks characteristic of the sibutramine standard spectrum mixed with silver colloids and potassium bromide aqueous solution (Figures 2i[d] and 2ii[d]) and do not exhibit peaks characteristic of other adulterants, which have been previously detected in these types of products. For analyzing suspect capsules containing sibutramine, the lowest detectable concentration (Cmin) was 5 μg/g for 1064 nm laser device and 1 μg/g for 785 nm laser device. The values in this range are low enough to detect sibutramine in dietary supplements. The supplements analyzed in this study were previously found to contain between 2–30 mg of sibutramine per capsule using high-pressure liquid chromatography with UV detection (HPLC-UV). The reported Cmins are 400 to 6000 times lower for the 1064 nm device and 2000 to 30,000 times lower for the 785 nm device relative to the concentration of sibutramine in individual capsules. The developed SERS method has its limitations, and API detection may depend on resonance, solubility, affinity for the metal surface, SERS cross section, or several of these factors.
The developed SERS method overcomes the sensitivity limitations of conventional Raman, and permits the low-level analysis of sibutramine typically observed in adulterated dietary supplements. Additionally, the developed SERS method in conjunction with other orthogonal techniques being used in the field can reduce the need to send IMF samples to regulatory laboratories while offering FDA the analytical data when needed to exercise its administrative destruction authority.
References
(1) https://www.fda.gov/news-events/congressional-testimony/oversight-federal-efforts-combat-spread-illicit-fentanyl-07162019
(2) H.R.6 - SUPPORT for Patients and Communities Act. https://www.congress.gov/bill/115th-congress/house-bill/6
(3) A. Lanzarotta, S. Kern, J. Batson, T.M. Falconer, M. Fulcher, K.W. Gaston, M.M. Kimani, L. Lorenz, F. Morales-Garcia, N. Ranieri, D. Skelton, M.D. Thatcher, V.M. Toomey,S. Voelker, and M.R. Witkowski, J. Pharm. Biomed. Anal. 203, 114183 (2021). https://doi.org/10.1016/j.jpba.2021.114183
(4) M. Fleischmann, P.J. Hendra, and A.J. Mcquillan, Chem Phys Lett. 26, 163–166 (1974). doi.org/10.1016/00092614(74)85388-1
(5) D.L. Jeanmaire and R.P. Van Duyne, J. Electroanal. Chem. Interfacial Electrochem. 84, 1–20 (1977). doi.org/10.1016/S00220728(77)80224-6
(6) A. Lanzarotta, L. Lorenz, J. Batson, and C. Flurer, J. Pharm. Biomed. Anal. 146, 420–425 (2017). https://doi.org/10.1016/j.jpba.2017.09.005
(7) M.M. Kimani, A. Lanzarotta, and J.S, Batson, J. Forensic Sci. 66(2), 491–504 (2021). doi.org/10.1111/1556-4029.14600
(8) A. Lanzarotta, M.D. Thatcher, L.M. Lorenz, and J.S. Batson, J. Forensic Sci. 65(5), 1443–1449 (2020). doi: 10.1111/15564029.14457.
(9) A. Lanzarotta, J. Batson, M. Thatcher, S. Kern, T. Falconer, M. Kimani, L. Lorenz, D. Skelton, K. Gaston, and S. Voelker, Technical and Logistical Considerations for Examining FDA-Regulated Products at International Mail Facilities using Handheld and Field-Portable Analytical Devices. Presented at the FDA Grand Rounds, November 14, 2019, White Oak, Maryland. Available online at https://www.fda.gov/science-research/fda-grand-rounds/technical-and-logistical- considerations-examining-fda-regulated-products-international-mail
(10) A. Lanzarotta, M.M. Kimani, M.D. Thatcher, J. Lynch, M. Fulcher, M.R. Witkowski, and J.S. Batson, J. Forens. Sci. 65(4), 1274–1279 (2020). doi: 10.1111/1556-4029.14287
(11) D.O.M. Bonsu, C. Afoakwah, and M.D. Aguilar-Caballos, Forensic Toxicol. 39, 1–25 (2021). https://doi.org/10.1007/s11419-020-00564-5
Martin M. Kimani (left), Adam Lanzarotta (right), and JaCinta S. Batson are with the Forensic Chemistry Center at the U.S. Food and Drug Administration, in Cincinnati, Ohio. Direct correspondence to: Martin.Kimani@fda.hhs.gov ●


AI-Powered SERS Spectroscopy Breakthrough Boosts Safety of Medicinal Food Products
April 16th 2025A new deep learning-enhanced spectroscopic platform—SERSome—developed by researchers in China and Finland, identifies medicinal and edible homologs (MEHs) with 98% accuracy. This innovation could revolutionize safety and quality control in the growing MEH market.
New Raman Spectroscopy Method Enhances Real-Time Monitoring Across Fermentation Processes
April 15th 2025Researchers at Delft University of Technology have developed a novel method using single compound spectra to enhance the transferability and accuracy of Raman spectroscopy models for real-time fermentation monitoring.
Nanometer-Scale Studies Using Tip Enhanced Raman Spectroscopy
February 8th 2013Volker Deckert, the winner of the 2013 Charles Mann Award, is advancing the use of tip enhanced Raman spectroscopy (TERS) to push the lateral resolution of vibrational spectroscopy well below the Abbe limit, to achieve single-molecule sensitivity. Because the tip can be moved with sub-nanometer precision, structural information with unmatched spatial resolution can be achieved without the need of specific labels.