Coherent Raman Imaging for Assessing Cutaneous Pharmacokinetics: Advancements and Outlook
At SciX 2021, we will be presenting our latest research on coherent Raman imaging to visualize and quantify cutaneous pharmacokinetics (PK). The field of coherent Raman imaging is fascinating because of the label-free, nondestructive nature in which molecular species can be quantified. Coherent Raman imaging allows for a vast range of biomedical applications to have the potential to go from benchtop to the clinic.
The cutaneous PK of active pharmaceutical ingredients (APIs) following topical formulation is an underinvestigated area of research. Measuring API concentration quantification after topical application is essential for PK and pharmacodynamic (PD) estimates because it is crucial in developing and evaluating topical products. In systemic drug delivery, plasma concentrations are used to estimate the target-site concentrations. However, for topically applied APIs, plasma concentrations in systemic circulation represent those APIs that are no longer at the action site. Thus, those APIs have little relevance to locally acting drug products. Ultimately, APIs exert their effects at the cellular and subcellular level, making microscale quantification of PK directly within treated skin essential.
There are several in vitro and in vivo methodologies (in vitro permeation testing, tape stripping, dermal open flow microperfusion, and dermal microdialysis) that are currently under investigation to assess the rate and extent at which a topical API becomes available at, or near, the site of action. However, each of these provides only macro-scale, or bulk, cutaneous PK (1–4). Although these methods are designed to yield cutaneous bioavailability (BA) and bioequivalence (BE) assessments, direct quantification of target-site API concentrations at the cellular level within skin would open the door to a deeper understanding of topical PK–PD relationships.
One such methodology to quantify cellular level API concentration is coherent Raman scattering imaging (CRI). CRI can monitor API cutaneous PK after topical application without the need for labels or alteration to the molecule. Therefore, CRI provides noninvasive concentration-time profiles (5). CRI modalities can tune into specific molecular vibrations of APIs to create chemically specific images at video-rate acquisition speeds in vivo (6). The two CRI modalities (coherent anti-Stokes Raman scattering (CARS) and stimulated Raman scattering (SRS)) are more commonly used because of their inherent sensitivity to Raman features that enable the identification of molecular species. In the context of cutaneous PK, CARS can be readily utilized to extract skin structure. SRS, whose signal intensity is linear with molecular concentration, allows for sensitive, selective, and specific quantification of APIs or inactive ingredients within skin stratifications.
Using CRI modalities has the potential to have a profound impact on the topical drug product industry. Innovator companies could have a better comprehension of their cellular level PK–PD relationship in addition to mechanistic cutaneous permeation knowledge. Generic companies would have an additional methodology to assess the cutaneous BE of their test product, thus passing along these savings to the patient. Many topical dermatological drug products do not have generics, which severely hampers the American public by making these medications cost-prohibitive (7).
In this study, we demonstrate the utility of CRI to identify cutaneous API localization by utilizing a machine learning approach that can provide insight into the tissue layer composition in which the API resides (that is, lipid-rich or protein–water-rich). The approach allows for the mechanistic insight of permeation pathways for specific formulation APIs and inactive ingredients, advancing cellular level cutaneous PK knowledge. The current limitations with CRI are addressed with suggestions on how these technological advancements can further improve the methodology for cutaneous BA and BE assessments.
CRI PK Groundwork
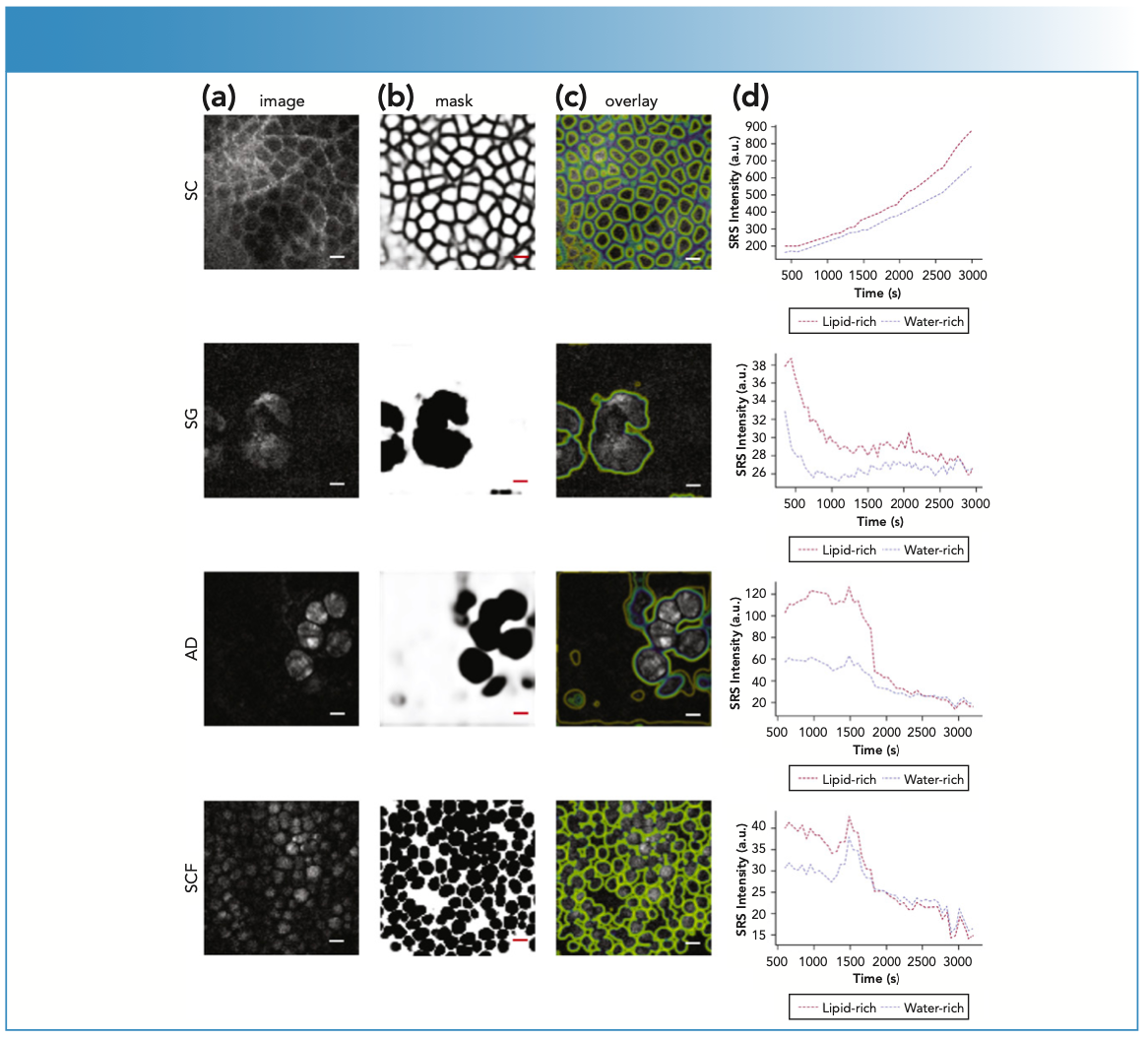
To address these questions, Feizpour and others recently utilized SRS to quantify ruxolitinib, a pan-janus kinase (JAK) inhibitor, within distinct nude mouse ear skin stratifications: the stratum corneum, sebaceous glands, adipocytes, and subcutaneous fat (8). A topical solution of transcutol containing ruxolitinib and a novel gel formulation containing ruxolitinib were applied to nude mouse ears and then imaged for 1 h. The images were then processed via an automated pipeline that comprised of a trained convolution neural network to precisely identify lipid- rich and water–protein-rich regions. The concentration-time data were extracted from the images and then analyzed via an open-source R package (NonCompart). This automated pipeline allowed the quantification and biodistribution estimation of ruxolitinib in each layer within the mouse ear. Previous SRS imaging attempts (9) were able to visualize and quantify the API penetrating through the stratum corneum and permeating deeper. However, this study was the first to demonstrate that SRS imaging has the capability to identify the API biodistribution specifically and which route of permeation (intracellular or intercellular) delivers an API (Figure 1). Although this proof-of-concept study demonstrates the capability of the technology, it was conducted on a laboratory CRI system affixed to a floating laser table. Translating this technological approach to a mobile, clinic-friendly CARS and SRS system is an active area of current research for our team.
FIGURE 1: Image segmentation was performed via layer specific U-Nets, which automatically identified the respective skin stratifications, namely stratum corneum (SC), sebaceous gland (SG), adipocytes (AD), and subcutaneous fat (SCF). Column (a) shows the SRS lipid images, column (b) displays the output of the U-Net analysis for each image, and column (c) plots these probability images as overlays over the original image. Column (d) displays relative permeation profiles patterns for the four layers differentiating lipid- from water- rich areas of the images for ruxolitinib in transcutol absorbed in ex vivo mouse skin. Bar = 20 μm. Adapted from the literature (8).
The Role of an Inactive Ingredient in Permeation
To build upon our previous research, we modified the composition of the topical solution to understand the impact of the inactive ingredient on the cutaneous PK and biodistribution in a nude mouse ear. As previously acknowledged, the inactive ingredients of the formulation play a vital role in delivering drugs to the desired cutaneous site (or sites) of action. We demonstrated in this study that the selection of inactive ingredients can alter the residence time in specific skin structures. The information obtained in these studies can help the topical communities develop targeted drug products to understand how various inactive ingredients elicit their effect. Because of the native skin signal in both the fingerprint regions (700–1800 cm-1) and high wavenumber regions (2700–3300 cm-1), these studies were limited to molecular species that had vibrations in the “silent region” (2000–2300 cm-1) of the Raman spectrum. Although carrying out silent-region imaging provides excellent concentration-time profile data, the handful of silent region active moieties (that is, alkyne, nitrile, or carbon-deuterium bonds) limits the number of APIs that can be imaged using this approach. New methodologies that take advantage of multiple vibrational spectral bands to detect and quantify APIs are under active development and promise to open this approach for use with a far greater number of API candidates.
The Future of CRI and Cutaneous PK
The work done by our group and others have allowed for CRI modalities to be utilized for cutaneous PK studies where API concentrations rapidly change. The CRI data provided here can yield critical target-site concentration information and dramatically increase cutaneous PK knowledge. As a result, the CRI data provided here helps us to efficiently model how the API penetrates the stratum corneum and permeates deeper into the skin. In addition, the ability to provide mechanistic insight into API permeation along with how the inactive ingredients of the formulation ultimately affect an APIs biodistribution has the potential to improve development of targeted drug products.
Although the work presented here provides optimism and excitement with what can be done, CRI has been primarily limited in two facets—the lack of mobile SRS imaging tools, and the lack of a general SRS method for spectral API quantification. As discussed above, creating a mobile imaging system for SRS remains an active area of research, requiring system integration of fiber lasers, epidetection schemes for interfacing with human skin, and full-stack image analysis methods to interpret the collected data. Although the immediate goal in this research is to create a new tool for topical BA and BE assessment, it goes without saying that such a system could be the foundation for a multitude of CRI methods, including clinical endoscopic tools.
Breaking out of the high wavenumber and silent regions has long been an important goal for the coherent Raman community. With advances in stimulated Raman scattering, tunable light sources, and detection methods driving advances, the coherent Raman community continues to see growth driven by innovation and technological improvements. However, ongoing technical advances in both optical hardware and analysis software will be required to fully translate new tools from the laboratory to clinical practice, with current research efforts serving as excellent foundational building blocks. Strong collaborations with major stakeholders in both the pharmaceutical industry and regulatory bodies will be needed to guide and implement these methods to make an actual impact on patient health.
Acknowledgments
The authors would like to thank the members of the Evans Group for the scientific discussion regarding the work presented within this text. The authors would also like to acknowledge LEO Science and Tech Hub in Boston and LEO Pharma in Denmark for sponsoring this project and providing the drugs and vehicles.
References
(1) M. Bodenlenz, K.I. Tiffner, R. Raml, T. Augustin, C. Dragatin, T. Birngruber, D. Schimek, G. Schwagerle, T.R. Pieber, S.G. Raney, I. Kanfer, and F. Sinner, Clin. Pharmacokinet. 56(1), 91–98 (2017).
(2) B.A. Kuzma, S. Senemar, T. Ramezanli, P. Ghosh, S.G. Raney, and G. Stagni, Eur. J. Pharm. Sci. 159, 105741 (2021).
(3) S.H. Shin, S. Thomas, S.G. Raney, P. Ghosh, D.C. Hammell, S.S. El-Kamary, W.H. Chen, M.M. Billington, H.E. Hassan, and A.L. Stinchcomb, J. Control. Release 270, 76–88 (2018).
(4) M. Hoppel, M. Tabosa, A. Bunge, M. Delgado-Charro, and R. Guy, AAPS J. 23(3), 1–11 (2021).
(5) C.L. Evans and X.S. Xie, Annu. Rev. Anal. Chem. 1, 883–909 (2008).
(6) C.L. Evans, E.O. Potma, M. Puoris’haag, D. Cote, C.P. Lin, and X.S. Xie, Proc. Natl. Acad. Sci. USA 102(46), 16807–16812 (2005).
(7) M.E. Rosenberg and S.P. Rosenberg, JAMA Dermatol. 152(2), 158–163 (2016).
(8) A. Feizpour, T. Marstrand, L. Bastholm, S. Eirefelt, and C.L. Evans, J. Investig. Dermatol. 11(12), 6864–6880 (2020).
(9) B.G. Saar, L.R. Contreras-Rojas, X.S. Xie, and R.H. Guy, Mol. Pharm. 8(3), 969–975 (2011).
Benjamin A. Kuzma is with the Wellman Center for Photomedicine, Massachusetts General Hospital, Harvard Medical School, in Boston.

Conor L. Evans is with the Wellman Center for Photomedicine, Massachusetts General Hospital, Harvard Medical School, in Boston. Direct correspondence to: evans.conor@mgh.harvard.edu


AI-Powered SERS Spectroscopy Breakthrough Boosts Safety of Medicinal Food Products
April 16th 2025A new deep learning-enhanced spectroscopic platform—SERSome—developed by researchers in China and Finland, identifies medicinal and edible homologs (MEHs) with 98% accuracy. This innovation could revolutionize safety and quality control in the growing MEH market.
New Raman Spectroscopy Method Enhances Real-Time Monitoring Across Fermentation Processes
April 15th 2025Researchers at Delft University of Technology have developed a novel method using single compound spectra to enhance the transferability and accuracy of Raman spectroscopy models for real-time fermentation monitoring.
Nanometer-Scale Studies Using Tip Enhanced Raman Spectroscopy
February 8th 2013Volker Deckert, the winner of the 2013 Charles Mann Award, is advancing the use of tip enhanced Raman spectroscopy (TERS) to push the lateral resolution of vibrational spectroscopy well below the Abbe limit, to achieve single-molecule sensitivity. Because the tip can be moved with sub-nanometer precision, structural information with unmatched spatial resolution can be achieved without the need of specific labels.