The Determination of Selenium in Shampoo by Flame Atomic Absorption Spectrometry
Application Notebook
The formal European Union analytical method to measure and regulate the concentration of selenium disulphide is by the determination of selenium via flame atomic absorption spectrometry.
Rebecca Price, Thermo Fisher Scientific
The formal European Union analytical method to measure and regulate the concentration of selenium disulphide is by the determination of selenium via flame atomic absorption spectrometry. The Thermo Scientific iCE 3000 Series Atomic Absorption Spectrometer offers a simple, quick, and accurate solution and meets the requirements of current EU legislation on cosmetic products.
Pityriasis capitis, more commonly known as dandruff, and seborrheic dermatitis, an inflammatory disease of the skin, are both treated with selenium disulphide. The active ingredient is typically delivered to the affected area through lotions and shampoos. In the case of anti-dandruff shampoo, prescription and nonprescription strength medications contain 2.5 % and 1 % selenium disulphide respectively.
One of the primary routes of human exposure to selenium disulphide is through the use of medicinal shampoos and lotions via dermal contact and inhalation. Although there is no substantial absorption through intact skin, broken or damaged skin can offer a route of entry to the body.
Selenium disulphide is considered to be a potential carcinogen when inside the body, but there is no conclusive evidence of any adverse effects when it is administered topically. Nevertheless limits have been set for the selenium disulphide content in shampoo and lotions.
The analysis of selenium disulphide in shampoo is contained in the 5th commission directive 93/73/EEC on the methods of analysis necessary for checking composition of cosmetic products. This directive was followed for the analysis of selenium disulphide in a range of medicinal shampoos, and this application note demonstrates how the iCE 3000 Series AA spectrometers can be used to meet the legislative requirements with ease.
Method
Selenium Identification
The presence of selenium can be identified by a characteristic yellow/orange color produced during a reaction with potassium iodide and urea. In the commission directive method, identification is performed before selenium determination, which is carried out by flame atomic absorption spectrometry.
Approximately 1 g of shampoo was weighed out into a digestion tube. 2.5 mL of concentrated nitric acid was added and then digested on a heated block digestor at 150 °C for 30 min. The sample was then quantitatively filtered through a filter paper and diluted to 25 mL with water. 2.5 mL of the filtrate was taken and boiled with 2.5 g of urea. After cooling, 1 mL of potassium iodide (10 % m/v) was added.
A positive indication for the presence of selenium was identified by a yellow/orange color, which darkens rapidly on standing. This is shown in Figure 1. The solution on the left of each picture is the control (blank) sample followed by 3 samples of anti-dandruff shampoo known to contain selenium.

Figure 1: Anti-dandruff shampoo samples after the addition of potassium iodide solution. (a) immediately after addition, (b) 5 min after addition, (c) 10 min after addition.
Selenium Determination
0.2 g of shampoo was accurately weighed into a digestion tube and 5 mL of concentrated nitric acid added. The sample was digested at 150 °C for 1 h and then allowed to cool. Quantitative filtration was performed through a filter paper (Whatman No 42 or equivalent, or 0.45 μm membrane filter) and the final volume made up to 100 mL with water. These solutions were then analysed for selenium using flame atomic absorption spectrometry as detailed below.
Standard and Reagent Preparation
Standard solutions of 10, 20, 30, 40, and 50 mg/L (ppm) were prepared by pipetting 1.0, 2.0, 3.0, 4.0, and 5.0 mL of the 1000 mg/L stock solution into individual volumetric flasks and then making up to 100 ml with 5 % nitric acid solution. Potassium iodide solution of 10 % m/v was prepared by dissolving 10 g of potassium iodide in 100 ml of water.
Flame Method
The lateral and rotational burner positions of the AA spectrometer were optimized along with the impact bead position using the "Optimize burner and nebulizer positions" wizard in the Thermo Scientific SOLAAR software. A wizard was also used to quickly and automatically determine the optimal gas flow and burner height positioning for selenium determination. The final method parameters are shown in Table I.
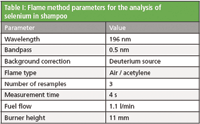
Table I: Flame method parameters for the analysis of selenium in shampoo
Aspiration of samples into the flame caused it to change from the typical blue color of a lean flame to orange. This is due to the large amount of sodium present in the samples, as sodium is contained in many preservatives and foaming agents found in shampoo. For this reason deuterium background correction was used. Background correction is stated as a requirement in the 5th commission directive (93/73/EEC).
Results and Discussion
A 5 point calibration curve for the analysis of selenium in shampoo was used as described in the method. Spiked samples were prepared to evaluate the recovery of selenium. This was done by adding 1 ml aliquots of the 1000 mg/L selenium standard to samples of shampoo prior to digestion. This is equal to a 10 mg/L addition in the final solution analyzed. All samples with a C suffix (as indicated in Table II) were spiked in this way. Results for the analyzed samples are shown in Table II.
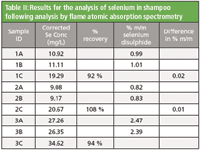
Table II: Results for the analysis of selenium in shampoo following analysis by flame atomic absorption spectrometry
The selenium disulphide content in the sample, in percentage by mass (% m/m) is calculated using the following formula:
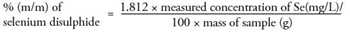
For a selenium disulphide content of 1 % (m/m) the method states that the difference between the results of two determinations carried out in parallel on the same sample should not exceed 0.05 % (m/m). For samples 1 and 2 the difference between two equivalent samples is 0.02 and 0.01 respectively, thus demonstrating that this analysis complies with the formal method guidance.
Sample 2 was a 1 % (m/v) branded shampoo and sample 3 was a 2.5 % (m/v) branded shampoo. The selenium disulphide content of sample 1 was not stated. As the method determination is calculated in % m/m and the concentration stated on the bottle was % m/v, a simple density measurement was used to convert the selenium disulphide content from m/m to m/v. This enabled a comparison of the measured concentration with that stated. As shown in Table III, for both samples the measured concentration was similar to that stated on the packaging.
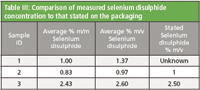
Table III: Comparison of measured selenium disulphide concentration to that stated on the packaging
Conclusion
The identification and determination of selenium disulphide as selenium in shampoo was carried out according to the method detailed in the 5th Commission Directive 93/73/EEC. Identification was performed by observation of a reactionary color change, while flame atomic absorption spectrometry was used to accurately determine selenium content. The determination was performed using a Thermo Scientific iCE 3000 Series AA Spectrometer which was optimized simply and quickly using the wizard-driven SOLAAR software. Deuterium background correction was used to ensure compliance with the legislation and spiked recoveries were used to verify the accuracy of the method.
References
Fifth Commission Directive 93/73/EEC on the methods of analysis necessary for checking composition of cosmetic products.
© 2010 Thermo Fisher Scienti?c Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scienti?c Inc. and its subsidiaries. Speci?cations, terms and pricing are subject to change. Not all products are available in all countries. Please consult your local sales representative for details.

Thermo Fisher Scientific
Madison, WI
tel. (800) 532-4752
Website: www.thermo.com

Thermo Fisher Scientists Highlight the Latest Advances in Process Monitoring with Raman Spectroscopy
April 1st 2025In this exclusive Spectroscopy interview, John Richmond and Tom Dearing of Thermo Fisher Scientific discuss the company’s Raman technology and the latest trends for process monitoring across various applications.
A Seamless Trace Elemental Analysis Prescription for Quality Pharmaceuticals
March 31st 2025Quality assurance and quality control (QA/QC) are essential in pharmaceutical manufacturing to ensure compliance with standards like United States Pharmacopoeia <232> and ICH Q3D, as well as FDA regulations. Reliable and user-friendly testing solutions help QA/QC labs deliver precise trace elemental analyses while meeting throughput demands and data security requirements.