LC–MS-MS-Based Strategies for the Targeted and Nontargeted Screening of Contaminants in Food, Environmental, and Forensic Samples
Liquid chromatography coupled to mass spectrometry (LC–MS) using electrospray ionization (ESI) is a powerful analytical tool for the analysis of a wide molecular weight range of polar, semivolatile, and thermally labile compounds.
Liquid chromatography coupled to mass spectrometry (LC–MS) using electrospray ionization (ESI) is a powerful analytical tool for the analysis of a wide molecular weight range of polar, semivolatile, and thermally labile compounds. Many versions of such instruments exist with the most popular being that of LC–MS-MS due to the extra degree of selectivity and sensitivity gained from being able to perform tandem MS experiments and to fragment molecular ions into characteristic product ions. While there are a range of different LC–MS-MS technologies available, including systems based upon quadrupole, time-of-flight (TOF), ion trap, and orbitrap technology, triple-quadrupole–based mass analyzers are the most popular for quantitation because when operated in multiple reaction monitoring (MRM) mode, the system works like a double mass filter, resulting in unmatched selectivity and sensitivity. As such, only molecules with the correct molecular ion filtered in Q1 and the correct product ion generated in the collision cell and filtered in Q3 can reach the detector and thus, very little noise and matrix interferences are detected. In addition, modern triple-quadrupole systems can measure MRM transitions very fast by accelerating generated product ions leaving the collision cell. This allows the use of very short dwell times, which is a requirement for the coupling of MS-MS with ultrahigh pressure liquid chromatography (UHPLC) to get enough data points across a given peak for accurate and reproducible quantitation.
While triple-quadrupole systems originally were used for single-compound quantitation by monitoring the abundance of a single MRM transition, there has been a move to use triple-quadrupole systems to screen for a larger number of compounds of interest in a single run. This is often referred to as multitarget screening (MTS) and is used to quickly screen for up to hundreds of targeted analytes, including pesticides, mycotoxins, and antibiotics in food samples, pharmaceuticals, and personal care products (PPCP) in water, and drugs of abuse and toxins in forensic samples, by monitoring hundreds of MRM transitions. Alternatively, MTS can be performed on high-resolution and accurate mass MS systems such as time-of-flight (TOF) analyzers, where the information of accurate molecular ions combined with the retention time is used to screen for targeted analytes. In both cases additional MS-MS information typically is needed to identify the detected compound with high confidence. Typically, the ratio of two MRM transitions (quantifier/qualifier) or a full-scan MS-MS spectrum searched against a spectral library is used to perform structural identification. Although such targeted screening methods are very fast, sensitive, and simple to implement, only known compounds are detected.
While use of MTS is now well accepted, there is a current movement to use nontargeted or general unknown screening (GUS). This can be explained by the recent high-profile food residue scandals, such as the detection of melamine in infant formula and the identification of the nonregistered pesticide isofenphos-methyl in strawberries that were not targeted as a potential contaminant and as such, did not show up until people became ill (1). Also, there is a growing need for GUS among environmental researchers to identify unknown persistent organic pollutants in the environment and for forensic scientists to investigate new and unexpected designer drugs.
While advancements in LC–MS-MS technology, including hybrid systems like triple-quadrupole linear ion trap and quadrupole TOF, now provide the ability to perform GUS on a routine basis, there are a number of tradeoffs that need to be considered when moving from a targeted to a nontargeted workflow. Because the GUS workflow does not use a target analyte list, compound detection is not based upon any a priori knowledge, including retention times and information on molecular and fragment ion. As such, the GUS workflow is less selective, making it impossible to detect compounds at concentrations as low as they can be detected using MTS. In addition, full-scan MS experiments used in a GUS workflow produce chromatograms very rich in information. The chromatograms obtained commonly contain thousands of ions from both compounds present in the sample as well as from the sample matrix itself. While the use of high resolution often is used to enhance selectivity by reducing interferences, the benefit of doing so is sample-dependent. Thus, powerful software tools are needed to explore the GUS data generated to find unknown compounds. This article, which is based upon the use of propreitary technology, contrasts MTS with GUS and illustrates the use of nontargeted peak-finding algorithms and statistical data analysis to find relevant information in complex samples followed by compound identification based upon MS-MS library searching.
Screening for Unknown Compounds in Food Samples Using a Nontargeted Peak Finding Algorithm in Comparison to Traditional Multitarget Screening
An MTS method for over 200 targeted pesticides was developed and applied to the analysis of spice and herbal extracts. Samples were extracted using a QuEChERS procedure (EN 15662:2007). Extracts were diluted 10 times before LC–MS-MS analysis to minimize possible ion suppression effects during ionization. LC separation was performed on an Ultra Aqueous C18 column (3 µm, 100 mm × 2.1 mm, Restek, Bellefonte, Pennsylvania) with a gradient of water–methanol and 10 mM ammonium acetate. This chromatographic setup provided adequate retention and excellent peak shape for all pesticides of the large panel investigated. Targeted pesticides were detected on a QTRAP 5500 triple-quadrupole linear ion trap LC–MS-MS system (AB SCIEX, Concord, Ontario) operated in MRM mode using the Scheduled MRM algorithm for best accuracy and reproducibility of quantitative results while monitoring more than 400 transitions. In parallel, samples were analyzed using a GUS workflow in which enhanced MS (EMS) scanning was used to detect nontargeted molecular ions, and enhanced product ion (EPI) scans were triggered automatically to be used for compound identification based upon searching against mass spectral libraries.

Figure1
A number of pesticides were quantified using the selective and sensitive MRM and identified based upon their MRM ratio. Example results of the analysis of a paprika powder are presented in Figure 1 and Table I.
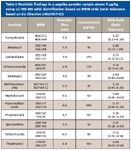
Table1
In parallel, the extracts were analyzed using the GUS method. Many hundreds of mass-retention time pairs were found in all sample extracts using the nontargeted peak-finding algorithm of Cliquid software (AB SCIEX). The EMS scan was used to find mass molecular ions of interest, and the automatically acquired EPI scans were used to identify detected compounds by library searching. Figure 2 shows an example of the detection and identification of carbendazim, which already was quantified during the MTS method. In addition, the GUS method found nontargeted ions such as mass 137 amu at 5.5 min. The signal was identified as nonivamide through MS-MS library searching. Nonivamide (pelargonic acid vanillylamide PAVA) is a capsaicinoid present in chili peppers but also manufactured synthetically. (The 137 ion is an in-source fragment of nonivamide, Figure 3.) The molecular ion of nonivamide 294 amu also was detected at the same retention time but with low sensitivity.

Figure2
Screening for Adulterants in Forensic Samples using Statistical Data Analysis
Another GUS application of LC–MS-MS is forensic analysis and crime scene investigation (CSI). In the following example, different street cocaine samples were analyzed using the EMS-EPI method described earlier on a 3200 QTRAP LC–MS-MS system (AB SCIEX) coupled to LC using a PFP Propyl column (5 µm, 50 mm × 2.1 mm, Restek) with a gradient of water–acetonitrile and 0.2% formic acid and 2 mM ammonium formate. Cocaine samples were diluted 1000 times and injected directly into the LC–MS-MS system.
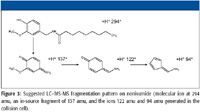
Figure3
The LC–MS-MS data were processed using principal components analysis (PCA) in the software used. PCA finds combinations of variables (LC–MS signals in this case) that explain most of the variance present in a data set. For each principal component, every sample has a score and every variable has a loading that represents its contribution to the combination. It is common to plot the scores and loadings of two principal components to visualize analytical results. Figure 4 shows the scores and loadings plots of the analysis of the cocaine samples.

Figure4
The scores plot clearly groups analyzed samples and the loadings plot helps to find characteristic marker ions. Automatically acquired MS-MS spectra were searched against a mass spectral library to identify the adulterants, the anesthetic procaine and vasodilator diltiazem, present in the cocaine samples (Figure 5). Information on cocaine adulteration and concentration is useful for crime scene investigation and to study illegal drug trade.

Figure5
Combination of Targeted and Nontargeted Screening for Pharmaceuticals and Personal Care Products (PPCP) in Environmental Samples
PPCP are environmental contaminants of growing concern. Typically, LC–MS-MS is used to quantify a few selected compounds and their metabolites. However, additional information on nontargeted PPCP is needed to properly assess the effects of such compounds on our environment. More than 70 water samples were collected in different cities and different countries from different types of waters, including drinking water, creeks, rivers, lakes, sea, and so on. Water samples were analyzed by LC–MS-MS in triplicates and in a randomized order. LC separation was performed using a 100 mm × 3 mm LUNA 2.5 µm C18(2)-HST column (Phenomenex, Torrance, California) with a gradient of water–acetonitrile and 0.1% formic acid. A 5500 LC–MS-MS system (AB SCIEX) was used to acquire MRM data to quantify 80 targeted PPCP and full-scan EMS data to detect other and unexpected environmental pollutants. Both MRM and EMS were used to acquire additional MS-MS data automatically for compound identification in a single run. High sensitivity MS-MS detection resulted in limits of quantitation as low as 1 ng/L (1 ppt), allowing direct injection analysis without time-consuming and costly cleanup.
The selective and sensitive MRM method was used to quantify and identify 80 PPCP in water samples. The targeted list contained pharmaceuticals, pesticides, and drugs of abuse, including important metabolites based upon method EPA 1694 and other chemicals of high concern. Figure 6 shows the quantitative results of benzoylecgonine, a metabolite of cocaine, in the studied water samples. As expected, benzoylecgonine was found in rivers running through major cities at concentrations of up to 200 ng/L, indicating the abuse of cocaine. The concentration of benzoylecgonine in water samples collected in less urban and wilderness areas was much lower with the exception of one creek and one lake in popular vacation destinations. The concentration of benzoylecgonine reflects the amount collectively excreted in urine and can be used to estimate drug consumption. Also, when analyzed over time, consumption habits can be investigated (2).

Figure6
All drinking water samples had a concentration of benzoylecgonine below 5 ng/L. Surprisingly, high levels of benzoylecgonine and other drugs, including painkillers and antidepressants, were found in a sample taken from the holy water basin of a church.
PCA was again used to study nontargeted compounds in the collected water samples (Figure 7). The scores plot clearly differentiates samples originating from different water types. River samples arrange depending upon urbanity. One drinking water sample was collected during flood season. The position of this sample in the scores plot indicates higher pollution of the drinking water produced from river bank filtrate.

Figure7
The loading plot indicates mass 192 amu at 7.7 min. Automatically collected MS-MS spectra searched against a library identified this signal as the insect repellent DEET (N,N-diethyl-m-toluamide) with an FIT value of 93%. The profile plot of DEET (Figure 7) shows that this compound is widespread in the environment and can be found even in remote areas with high recreational use such as Algonquin Provincial Park, a popular canoeing destination in Ontario, Canada, the Rocky Mountains in Colorado, and in the Alps, Switzerland.
Conclusions
LC–MS-MS is a powerful analytical tool for the analysis of a wide polarity and molecular weight range of compounds. Advances in mass analyzer technology now provide the ability to perform MTS and GUS on a routine basis. While GUS is attractive, there is currently a trade-off in terms of sensitivity and the complexity of data achieved by moving from a targeted to nontargeted approach. As such, powerful software tools, including nontargeted peak-finding algorithms, statistical data-mining tools, and MS-MS library search capabilities are required for this challenging task, as seen from the unexpected chemicals detected in the studies presented. This work indicates that there is value in employing both workflows to be able to meet required detection limits as well as to limit the complexity of data analysis.
André Schreiber and Nadia Pace are with Applied Biosystems, Concord, Ontario, Canada.
References
(1) St. Lehotay et al., TrAC 27/11, 1070–1090 (2008).
(2) S. Castiglioni et al., Mass Spectrom. Rev. 27, 378–394 (2008).

High-Speed Laser MS for Precise, Prep-Free Environmental Particle Tracking
April 21st 2025Scientists at Oak Ridge National Laboratory have demonstrated that a fast, laser-based mass spectrometry method—LA-ICP-TOF-MS—can accurately detect and identify airborne environmental particles, including toxic metal particles like ruthenium, without the need for complex sample preparation. The work offers a breakthrough in rapid, high-resolution analysis of environmental pollutants.
The State of Forensic Science: Previewing an Upcoming AAFS Video Series
March 10th 2025Here, we provide a preview of our upcoming multi-day video series that will focus on recapping the American Academy of Forensic Sciences Conference, as well as documenting the current state of the forensic science industry.
Previewing the American Academy of Forensic Sciences Conference
February 14th 2025This year, the American Academy of Forensic Sciences Conference is taking place from February 17–22, 2025. We highlight the importance of spectroscopy in this field and why we’re covering the conference this year.