Using the Full Spectrum for Raman: From UV to NIR
Application Notebook
The "inelastic scattering of light," or Raman effect, was observed in practice for the first time in 1928 by C.V. Raman for which he was awarded the Nobel Prize in 1930.
Raman Scattering
The "inelastic scattering of light," or Raman effect, was observed in practice for the first time in 1928 by C.V. Raman for which he was awarded the Nobel Prize in 1930. It is only in the last two decades, however, that Raman spectroscopy has begun to realize its potential as an almost universally applicable analytical technique from materials and life sciences applications to point of care analysis. This is primarily thanks to the availability of compact laser sources, high sensitivity cameras, and high resolution compact spectrometers.
Wavelength is Key
The physical basis of the technique offers huge flexibility and advantages in comparison with its sister technique, infrared spectroscopy, but at the same time presents a key challenge: the excitation needs to be highly (i) monochromatic (the Raman bands have the same shape as the light source) (ii) collimated and (iii) intense (due to the low probability of inelastic scattering, < 1 in 106 photons). Hence, it is the advent of lasers that has brought Raman spectroscopy — literarily — into the field.
In selecting the correct laser, the wavelength is important and depends on the application. For example, fluorescence is usually much stronger than the Raman scattering signal but unlike the fluorescence, the Raman scattering signal is observed even when exciting at wavelengths outside the absorption spectrum. Figure 1 illustrates this by showing that the fluorescence that swamps the Raman spectrum of a boron based fluorophore when the Raman spectrum is recorded at 785 nm (where absorption/fluorescence competes with the Raman process) is completely absent in the spectrum obtained at 1064 nm. Thus highlighting the importance of selecting the right excitation wavelength.
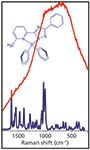
Figure 1: Near-IR dispersive Raman spectrosocpy. Comparison of the spectrum of a solid sample of an organic fluorophore at 785 nm (red) with that obtained at 1064 nm (blue). Excitation with a Cobolt Rumba⢠1064 nm (100 mW at sample), detection 4 by 5 s exposure, Andor idus163 spectrograph with an iDus-InGaAs detector.
DPSS Lasers for Raman Spectroscopy
Until recently, ion gas lasers (Ar, He, HeCd, and Kr) have been the first choice for Raman spectroscopy. However, the ever increasing number of wavelengths available with continuous wave diode pumped solid state (DPSS) lasers together with the high average powers (> 1 W) and compact footprint means that multi-wavelength Raman spectroscopy can be implemented as a turnkey low maintenance solution in any laboratory as well as in field portable applications. For example, Cobolt DPSS lasers qualify as excellent Raman excitation sources, thanks to their extremely narrow linewidth (<1 MHz), excellent wavelength stability, and high level of spectral purity (-60 dB).
Raman Scattering Is Inherently Weak — Or Is It?
Although it is true that the relative number of inelastically scattered photons is vanishing small, this does not mean that Raman spectroscopy cannot be used to detect compounds at low concentrations (< 1 mM) or that spectra cannot be obtained rapidly. When the laser used is close to or on the same wavelength as a compound absorbs light (that is, is in resonance) then the Raman signal from that compound can be enhanced by up to 104 times. An example of the resonance Raman effect is shown in Figure 2; by choosing 355 nm rather than 473 nm laser excitation the observed Raman signals are much stronger, thus providing improved resolution.
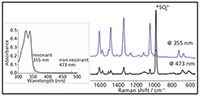
Figure 2: Raman spectra of a copper(II) complex (1 mM in water) obtained using 355 nm (Cobolt Zoukâ¢, 10 mW) and at 473 nm (Cobolt Blues⢠50 mW). Because 355 nm is close to the electronic absorption of the copper(II) complex, the spectrum is much more intense than would be expected due to resonance enhancement.
Conclusion
With the current availability of high performance DPSS lasers offering a broad range of wavelengths from the UV to the NIR, the applications for Raman spectroscopy are only just beginning to be fully explored.
References
(1) P. Dijkstra, D. Angelone, E. Taknishnikh, H.J. Wörtche, E. Otten, and W.R. Browne, Dalton 10.1039/C4DT01393J (2014).
(2) W.R. Browne and J.J. McGarvey, Coord. Chem. Rev. 251, 454–473 (2007).
Cobolt AB
Vretenvägen 13,17154 Solna, Sweden
tel. 46 8 5 45 912 30, fax 46 8 5 45 912 31
Website: www.cobolt.se

Thermo Fisher Scientists Highlight the Latest Advances in Process Monitoring with Raman Spectroscopy
April 1st 2025In this exclusive Spectroscopy interview, John Richmond and Tom Dearing of Thermo Fisher Scientific discuss the company’s Raman technology and the latest trends for process monitoring across various applications.
A Seamless Trace Elemental Analysis Prescription for Quality Pharmaceuticals
March 31st 2025Quality assurance and quality control (QA/QC) are essential in pharmaceutical manufacturing to ensure compliance with standards like United States Pharmacopoeia <232> and ICH Q3D, as well as FDA regulations. Reliable and user-friendly testing solutions help QA/QC labs deliver precise trace elemental analyses while meeting throughput demands and data security requirements.