Using the Orbis Micro-XRF Spectrometer to Study the Microstructure of Ancient Roman Seawater Concrete
Application Notebook
Ancient Roman builders designed maritime concrete harbor structures to remain intact in the aggressive seawater environment for very long periods of time.
Ancient Roman builders designed maritime concrete harbor structures to remain intact in the aggressive seawater environment for very long periods of time. Modern concrete engineers continue to deal with the challenges of exposure to chlorine ions, via either seawater or road salt. When chlorine ions penetrate into modern cement-based concrete they corrode the steel reinforcement, which ultimately can cause failure of the concrete structure. In contrast, the ancient Roman maritime structures have retained their structural integrity for 2000 years, despite having been partially or fully immersed in seawater since their construction. The concrete is composed of a volcanic ash-hydrated lime mortar that binds decimeter-sized chunks of volcanic tuff or limestone, shown by cores drilled by the ROMACONS research group from 11 different Roman harbors (1). There is no steel reinforcement. One key raw material is pumiceous volcanic ash quarried around Pozzuoli Bay in the Gulf of Naples, which reacts pozzolanically with lime and seawater to produce enduring cementitious hydrates in the mortar. Romans mastered the formulation and installation of maritime concrete in harbor structures in Pozzuoli Bay and the central Italian coast, most of which remain durable and intact to this day. The formulation of the concrete is theorized to have then spread through the Mediterranean area by the movement of Roman engineers and engineering manuals, which facilitated construction of concrete harbors. Analyzing these concrete samples for their elemental distribution with modern techniques like micro-X-ray fluorescence (micro-XRF) can give insight to the unique properties and processes that contribute to its longevity.
Characterization Technique
Micro-XRF is an elemental analysis technique that focuses an X-ray beam to generate characteristic elemental lines from the sample, with detection starting at sodium (Na). The X-rays are focused by means of total internal reflection through a series of glass capillaries, allowing high flux to be maintained at very small beam sizes. An X-Y-Z moveable stage allows for elemental distribution maps. For the measurements in the Roman mortar from Pozzuoli Bay, the EDAX Orbis PC-SDD spectrometer was used, which utilizes a 50 W Rhodium anode X-ray tube, a 30 µm diameter (FWHM at MoKα) X-ray beam (normal to sample), and a silicon-drift detector.
Using micro-XRF provides a number of advantages, particularly with historical and potentially high-value items. It is nondestructive, meaning the exciting X-ray beam does not damage the sample and the sample can be measured "as is." To contrast with some common characterization techniques for concrete, micro-XRF does not require coating the sample to prevent charging as with scanning electron microscopy-energy-dispersive spectroscopy (SEM-EDS). Techniques that utilize laser ablation, such as laser-ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) and laser induced breakdown spectroscopy (LIBS), will damage the sample, and are not acceptable in a case where the analyst wants to make high resolution compositional maps. Keeping the sample undamaged and intact as much as possible is especially important if the analyst intends to use multiple analytical techniques.
Another advantage to micro-XRF is that the sensitivity is 10 to 100 times superior to EDS for higher energy elements (that is, transition metals and above), typically reaching at least 50 ppm with a 30 µm beam diameter. Detection of chlorine, a key element to failure analysis of modern cement-based concrete with steel reinforcements, can reach about 15 ppm with optimized measuring conditions. Because lighter or organic elements, such as C, N, and O are not of interest in this analysis, EDS is not ideal. Another factor is the large sampling area, which in this example (Figure 1) is approximately 4.6 × 3.6 mm. Micro-XRF mapping lends itself well to larger mapping areas (mm scale or larger) as contrasted with EDS, which maps on a much smaller scale (µm to mm) and requires extremely long mapping times for satisfactory spatial resolution.
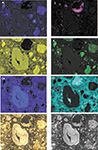
Figure 1: Orbis micro-XRF imaging panel of ancient Roman marine mortar submerged in Pozzuoli Bay since ~55 BCE (1,2,3). The color video image is in the lower left corner and the gray-scale video image used for elemental overlays is in the lower right corner. Elemental X-ray maps are shown in the top 6 images.
Sample
The concrete sample is a polished thin section, 0.3 mm thick, permeated with epoxy resin to maintain structural integrity of the thin section. In this case, the mounting epoxy contains Cl which causes some interference in the Cl elemental map and highlights the importance of selecting an epoxy that does not contain elements in significant concentrations that are common to the study of concrete, such as Cl, in order to avoid signal interferences. The analytical area was approximately 16.6 mm2, and a 30 µm diameter X-ray beam collected 512 × 400 points in the X and Y direction, respectively, at a dwell time of 500 ms per point.
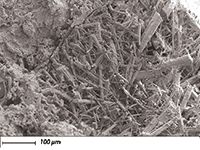
Figure 2: SEM-BSE image of chlorine-rich hydrocalumite crystals in relict void.
Results
The Orbis micro-XRF mapping in Figure 1 shows the characteristic mortar microstructures of Roman marine mortar (2,3). High silicon, aluminum and potassium occur in relicts of pumiceous volcanic ash from Campi Flegrei volcano (3); relict lime clasts, now mainly transformed to Al-tobermorite crystals ([Ca4 (Si5.5Al0.5 O17H2)]Ca0.2·Na0.1·4H2O), contain higher calcium and silicon; chlorine is sequestered in hydrocalumite crystals (Ca4Al2 (OH)12 (ClCO3OH)2 ·4H2O) (Figure 2) which partially fill the relict cavity and surround a relict lime clast. The relict cavity is filled with residual epoxy as shown by the Cl signal (Figure 1, point A). The brighter spots in the Cl map of the pore show the location of the hydrocalumite crystals which correlate with bright spots in the Ca and Al maps. See Figure 3a which shows the Cl map in a pseudo color scale to highlight the four bright spots within the Cl background signal of the epoxy-filled cavity. Full spectral data is collected at each map pixel and can be used to show the corresponding Al, Ca, and Cl signals from one of the hydrocalumite crystals in the cavity (Figure 3b). Sulfur is concentrated in ettringite crystals (Ca6Al2 (SO4)3 (OH)12 ·26(H2O) in relict voids as can be seen in the sulfur map of Figure 1. The sequestration of Cl- and SO42- anions in crystalline microstructures in Roman maritime concretes is in contrast to modern concrete formulations, where these anions typically participate in secondary expansion reactions which lead to the loss of structural integrity in modern concretes.

Figure 3: (a) Cl elemental map in pseudo coloring scale to highlight the hydrocalumite crystals in the epoxy-filled cavity; (b) XRF spectrum recalled from a bright spot within the epoxy-filled cavity of the Cl elemental map. The presence of Al, Cl, and Ca signals corroborates the identification of a hydrocalumite crystal.
Conclusion
The use of the Orbis micro-XRF spectrometer to analyze the microstructure of Roman maritime mortar results in high resolution elemental mapping of a large area that shows the complex cementitious fabric of this highly durable material. A 30 µm poly-capillary X-ray optic focuses a high intensity X-ray beam onto the sample. Full spectral data are collected at each mapping pixel so that the analyst can recall spectral data from the maps to confirm the identification of microstructures within the map.
References
(1) C. Brandon, R.L. Holhlfelder, M.D. Jackson, and J.P. Oleson, Building for Eternity: the History and Technology of Roman Concrete Engineering in the Sea (Oxbow Books, Oxford, England, 2014).
(2) M.D. Jackson et al., "Cement Microstructures and Durability in Ancient Roman Seawater Concretes," Historic Mortars: Characterisation, Assessment and Repair (RILEM Book series 7, 2012).
(3) M.D. Jackson et al., Am. Mineral.98, 1669–1687 (2013).
EDAX, Inc.
91 McKee Drive, Mahwah, NJ 07430
tel. (201) 529-4880, fax (201) 529-3156
Website: www.edax.com

Thermo Fisher Scientists Highlight the Latest Advances in Process Monitoring with Raman Spectroscopy
April 1st 2025In this exclusive Spectroscopy interview, John Richmond and Tom Dearing of Thermo Fisher Scientific discuss the company’s Raman technology and the latest trends for process monitoring across various applications.
A Seamless Trace Elemental Analysis Prescription for Quality Pharmaceuticals
March 31st 2025Quality assurance and quality control (QA/QC) are essential in pharmaceutical manufacturing to ensure compliance with standards like United States Pharmacopoeia <232> and ICH Q3D, as well as FDA regulations. Reliable and user-friendly testing solutions help QA/QC labs deliver precise trace elemental analyses while meeting throughput demands and data security requirements.