Efficient Discrimination of Some Moss Species by Fourier Transform Infrared Spectroscopy and Chemometrics
Fourier-transform infrared (FT-IR) spectroscopy with the attenuated total reflectance (ATR) technique was used to classify 14 samples (11 species) from three moss families (Mielichhoferiaceae, Bryaceae, and Mniaceae). The FT-IR spectra ranging from 4000 cm−1 to 650 cm−1 of the 14 samples were obtained. To group the spectra according to their spectral similarity in a dendrogram, cluster analysis and principal component analysis (PCA) were performed. Cluster analysis combined with PCA was used to give a rough result of classification among the moss samples. However, some species belonging to the same genus exhibited very similar chemical components and similar FT-IR spectra. Discrete wavelet transform (DWT) was used to enhance the differences among species with similar chemical components and FT-IR spectra. Three scales were selected as the feature-extracting space in the DWT domain. Results showed that FT-IR spectroscopy combined with chemometrics was suitable for a systematic research classification of bryophytes.
Bryophytes (the traditional name for any seedless plant) are found everywhere in the world except for in the sea, with many species having broad geographical locations that span several continents. With over 12,000 species recognized worldwide, the bryophytic subset of mosses are one of the world richest nonvascular land plants (1). Mosses are usually concentrated in groups and occur in loose tufts. While a minority of bryophytes are annuals, the large majority of them are perennials. The lack of true veins have restricted the size of mosses to only a few inches in height. The persistent photosynthetic phase of the moss life cycle is the gametophyte generation. Spores are released from a sporophyte capsule at certain times. Moss stems are prostrate or suberect and moss leaves commonly exhibit a spiral phyllotaxy or distichous and tristichous arrangements, while moss rhizoids are often branched (2).
As in most groups of plants, moss species are typically defined morphologically. Mosses show extensive morphological and anatomical diversification in both gametophyte and sporophyte organizations (2). The taxonomic and phylogenetic relationships among the families of Mielichhoferiaceae, Bryaceae, and Mniaceae have given rise to much controversy in recent years (3). Studies using nuclear, plastid, and mitochondrial sequences showed that many genera of Mielichhoferioideae and Bryaceae are polyphyletic. For instance, some species of the genus Pohlia, clearly belong to Mniaceae rather than Bryaceae (4–7). In addition, some mosses among the three families are known for their medicinal properties, for instance, Rhodobryum roseum has long been used as medical plants. Rhodobryum roseum extractives, such as ursolic acid, flavonoids, and alkaloids, have been used in cardiac study, and the extracts are more frequently used in medical research (8).
Using traditional phytotaxonomic methods, many species of Mielichhoferioideae and Bryaceae have been distinguished by minor differences in the gametophyte, peristome, and asexual structures, which are quite difficult to be differentiated. Fourier-transform infrared (FT-IR) spectroscopy is an alternative method for species differentiation. FT-IR has already been used to identify medicinal plants, bacteria, fungi, and other microorganism (9–14). Although FT-IR spectroscopy has made a significant contribution to plant classification, reports on moss taxonomy using FT-IR are still rare. In this study, our aim is to differentiate the circumscription of the Mielichhoferiaceae, Bryaceae, and Mniaceae families, so as to provide basis for the development of a classification system for bryophytes.
FT-IR is an original spectroscopic technique used to differentiate between species (15,16). The FT-IR spectrum of a compound can express a unique “fingerprint,” which enables FT-IR spectroscopy to be used in the classification of different samples or identification of unknown samples (17). However, in many practical problems, judgments cannot be quickly and accurately made by purely relying on FT-IR spectroscopy analysis (18). Chemometric methods combined with FT-IR spectroscopy can compensate for the errors inherent in a single FT-IR spectral analysis (19).
To classify the spectra, different multivariate methods, such as cluster analysis (CLA) and principal component analysis (PCA) were applied (20). CLA and PCA are two multivariate analysis techniques useful for FT-IR spectrum analysis (21). CLA and PCA can be used to identify natural clustering patterns and groups of samples based on spectral similarities among the samples. CLA and PCA are widely recognized as powerful tools in obtaining information about spectral relationships within a data set (22). To classify and compare the spectra of species from different genera, FT-IR spectra combined with multivariate methods are necessary.
Wavelet transform (WT) is another useful tool that can be used for a variety of signal processing applications. WT was developed to enhance the discrimination of variable signals having different frequency features (23). WT allows greater information to be extracted from spectral signals, and the resulting WT coefficients can be considered to be the key characteristics of a spectrum signal. Therefore, a few features can be used to represent the major spectral information following a wavelet function transformation. WT can be considered as one of the most useful and efficient chemometric methods (24).
Discrete WT (DWT) is used to decompose a signal by using filters to extract interesting frequency resolution components within the signal. DWT possesses compact support in both time and frequency domains (25). It is a signal-processing tool that is used in many engineering, scientific, and mathematical applications. DWT is used to analyze the signal at different frequency bands with different resolutions by decomposing the signal into a coarse approximation and also detailed information (26).
DWT originated from the discretization of continuous WT (CWT), and the common discretization is dyadic. CWT is provided by:
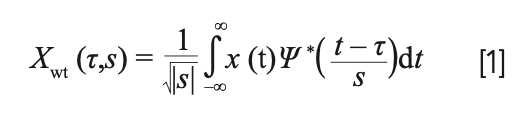
where X(t) is the signal to be analyzed and Ψ*(t) is the mother wavelet or the basis function. All of the wavelet functions used in the transformation are derived from the mother wavelet through translation and scaling.
In CWT, the signals are analyzed using a set of basic functions, which are related to each other by simple scaling and translation. In the case of DWT, a time-scale representation of the digital signal is obtained using digital filtering techniques. The DWT is derived from the discretization of CWT (τ, s) and the most common discretization is dyadic.
DWT is provided by:
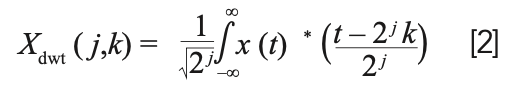
where τ and s are replaced by 2j and 2jk. The DWT is computed by successive low-pass and high-pass filtering of the discrete time-domain signal. This computation is called the Mallat algorithm or Mallat-tree decomposition. Its significance is in the manner it connects the continuous-time multiresolution to discrete-time filters. With this approach, the time resolution becomes arbitrarily good at high frequencies, whereas the frequency resolution becomes arbitrarily good at low frequencies.
In this study, the classification of 14 samples of mosses (11 species) was studied. The present study aimed to evaluate the potential use of FT-IR spectroscopy combined with CLA and PCA to differentiate between the 14 samples of mosses (11 species). DWT was used to investigate the chemical fingerprint variability among the species.
Materials and Methods
Materials
14 samples of mosses (11 species) were collected from three different sites. Four samples were collected from Sichuan Province and ten samples were collected from Hebei Province, China, in August 2016. The geographic coordinates and altitudes of the sites of the samples are shown in Table I. The environmental conditions and population sizes of the sample plants were similar. The voucher specimens were deposited in the Herbarium of Hebei Normal University.
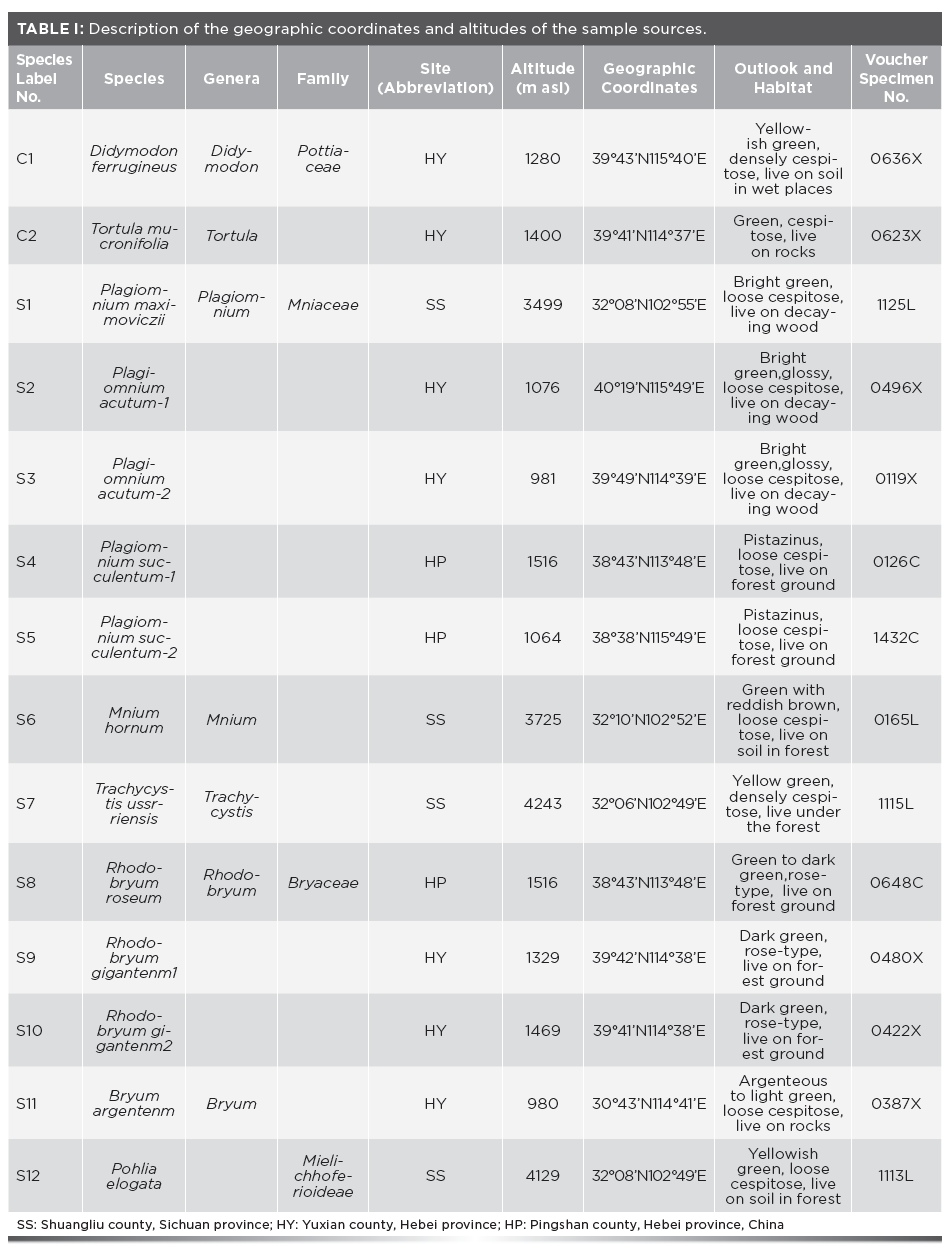
To avoid problems caused by the influence of water on FT-IR spectra, a vacuum freeze drying machine (FD-1) was used for freeze drying. The condensation temperature was -52 oC and the vacuum was 5 Pa. All samples were dried and ground into a fine powder using an agate mortar and sieved through a 200 mesh screen (75 μm).
Spectral Measurements
The FT-IR spectra (ranging from 4000 cm−1 to 650 cm−1, 4 cm−1 resolution, 32 scans) were obtained with a ZnSe attenuated total reflectance (ATR) accessory (PIKE Technologies MIRacle) combined with an FT-IR spectrometer Bruker Optics Vertex70 equipped with a DigiTect detector that can prevent external signal disturbance and provide the highest signal-to-noise ratio. After grinding, 6 mg of each powdered sample was directly placed about 2.54 mm2 on the center of the ZnSe crystal plate. All samples were pressed using the same mechanical pressure, and the FT-IR spectra was obtained. The FT-IR spectra of the 14 samples (11 species) of mosses were automatically baseline corrected. Each of the species sample was measured three times and the averaged spectrum was obtained for further analysis. Baseline and mean-centering correction were used to remove sources of variability.
Precision, Repeatability, and Stability Test
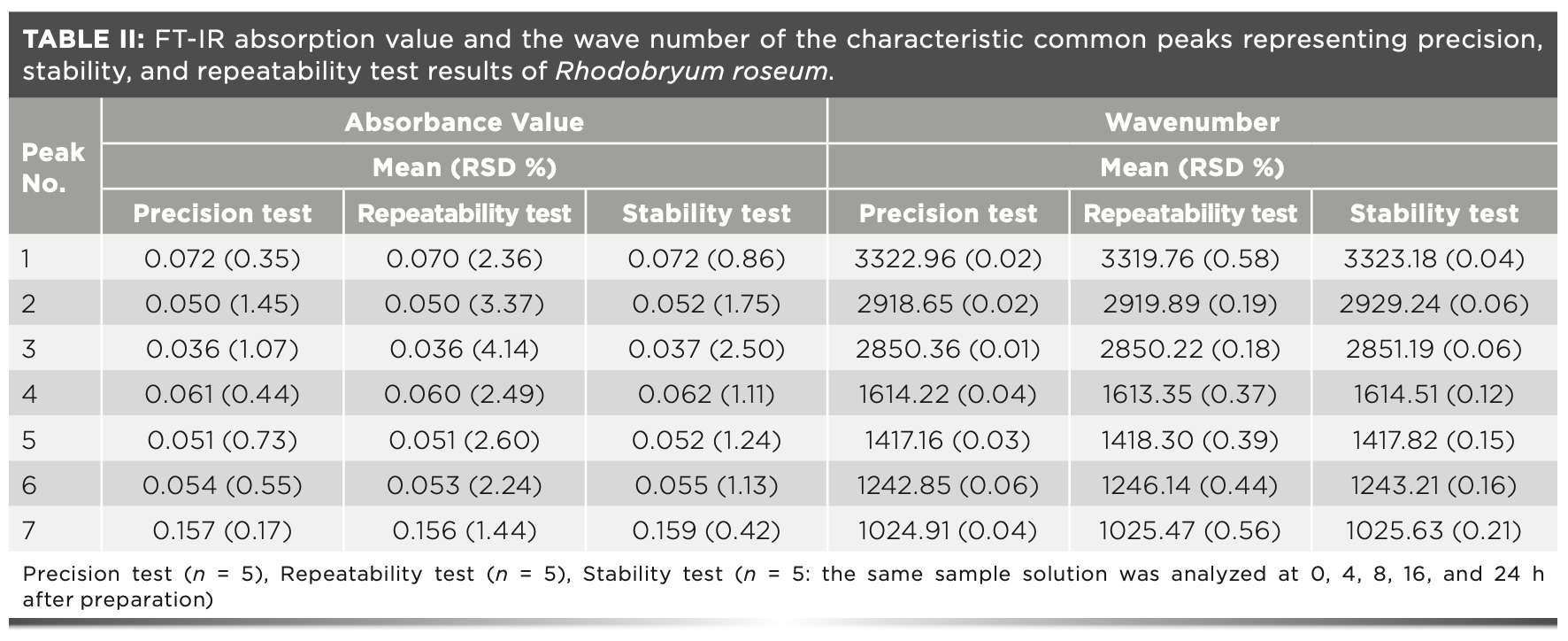
Sample S8 (Rhodobryum roseum) was used to validate the method. The precision test was conducted using replicate records (5×) of the same sample. The repeatability test was analyzed by gathering the data of five independently prepared samples of the same species. The stability test was determined by five records of one sample solution during 24 h. The mean value relative standard deviation (RSD) of the absorbance and wavenumber for common peaks for each test was calculated.
Data Analysis
The FT-IR spectra of the samples were recorded and found to be within the range of 4000 to 650 cm−1. Spectral data were calculated with the use of OPUS version 6.5 software (Bruker). CLA and PCA were performed using R ver. 3.6.1 (<www.r-project.org>). Cluster analysis is an undirected and unbiased statistical method that is used to analyze spectroscopic information (27). Cluster analysis is also used to sort the FT-IR spectra into similar sets or groups. Satisfactory results using FT-IR spectra of complex biological molecules have been obtained using Ward’s algorithm, Euclidean distances, or correlation coefficient calculation as distance metrics (28,29). In the present study, 14 samples (11 species) of mosses are selected for clustering. We chose 12 absorption peaks in the range of 4000 to 650 cm−1 for cluster analysis. The Euclidean distance, within-groups linkage and Pearson correlation were used to construct the circle dendrogram.
PCA is a multivariate statistical data reduction method that transforms an original set of variables into a new set of uncorrelated variables called principal components (PCs) (30). PCA also provides information on the major spectral components, wherein dominant factors determine differences among samples (31,32). PCA is used to extract the important features of a correlation matrix in terms of PCs. Only a few PCs are usually required to explain the majority of observed variance. PCA can be used as a chemometric method for FT-IR analysis. The analysis can be presented as either two-dimensional (2D, two PCs) or three-dimensional (3D, three PCs) scatter plots (33). In this study, the selected absorption peaks were used for PCA, and the factor loading was plotted.
DWT can solve numerous difficult problems that the Fourier transform cannot. Hence, DWT is also known as the “math microscope.” DWT was performed using MATLAB 7.1 software. Daubechies wavelet has better exploration ability for signal singularity, which served as the preferred analysis wavelet (34). One-dimensional stationary DWT was performed on different samples.
Results and Discussion
Validation of the Method
Precision Test
The precision test was conducted by obtaining replicate measurements of the same sample (S8) for five times within an hour. The RSD of the FT-IR absorbance value of the common peaks were ≤1.45%. The RSD of the wavenumber of the common peaks were ≤0.06%. The results are shown in Table II.
Repeatability Test
The repeatability of the method was assessed by analyzing five independently prepared samples (S8) of the same species (Rhodobryum roseum) using the same method. The RSD of the FT-IR absorbance value of the common peaks were <4.15%. The RSD of the wavenumber of the common peaks were ≤0.58%. The results are shown in Table II.
Sample Stability Test
Sample stability was determined using sample S8. The S8 sample powder was stored at room temperature for 24 h. The RSD of the FT-IR absorbance value of the common peaks were ≤2.5%. The RSD of the wavenumber of the common peaks were ≤0.21%. The similarity of results indicated that the sample of the same species remained stable within 24 h. The results are shown in Table II.
The running results show that the FT-IR absorbance value and the wavenumber of the characteristic common peaks of the same species are stable. Therefore, the method exhibits good repeatability, is reliable and can be applied in the analysis of other moss samples.
FT-IR Analysis
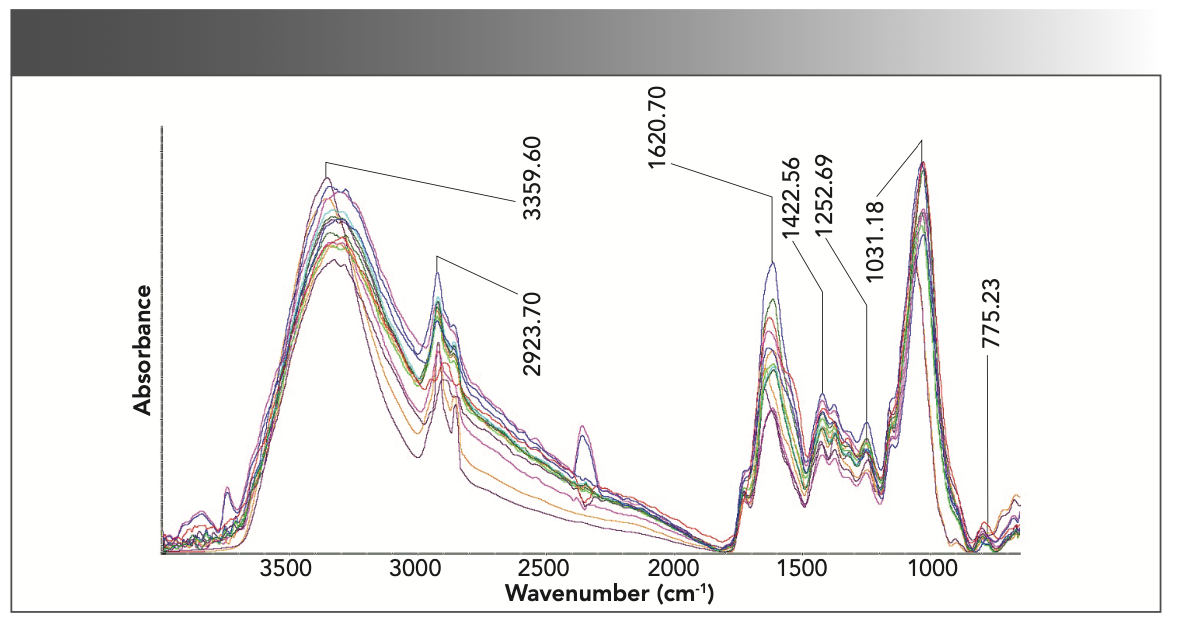
The FT-IR spectra of the 14 samples (11 species) of mosses were recorded including seven Mniaceae samples, 4 Bryaceae samples, one Mielichhoferioideae sample and two Pottiaceae samples (control group) (Figure 1). Most of the peaks in the FT-IR spectra obtained from the 14 samples are shown in Figure 1. Most of the peaks represent major functional groups, which show the comprehensive information on the protein, carbohydrate, fibrin, and lipid components. The FT-IR spectra comparison could provide information on different samples. Hence, the region between 3500 to 3000 cm−1 presents a broad band centered at approximately 3358 cm−1, which corresponds to absorption because of the stretching of O–H and N–H bands (Figure 1). A sharp peak at 2920 and 2852 cm−1 was attributed to the presence of polysaccharide, lipid, and carbohydrates (C–H stretch). The peak at 1620 cm−1 was attributed to the absorbance of amide (C=O bend). A second amide vibration was shown at 1420 cm−1 (C–H stretch), followed by an amide peak at 1260 cm−1 (C–N stretch). The peak at 1030 cm−1 can be attributed to oligosaccharides, glycoprotein, and cellulose (C–O stretch). The peaks from 1200 to 650 cm−1 can be attributed to the absorbance of low-molecular weight carbohydrates, polyols, and monosaccharides; this region is characterized as the fingerprint region (35).
FIGURE 1: ATR-FT-IR spectra obtained between 4000 to 650 cm−1 for the 14 samples. The main peaks are labeled at the top of the spectra.
Cluster Analysis
The FT-IR spectra from the different species of mosses exhibit similar absorbance. Specific differences are difficult to distinguish by experience. Therefore, we chose to utilize multivariate statistical methods to analyze the absorption bands. Cluster analysis was conducted to investigate the relationship between species. We chose 12 absorption peaks within the range of 4000 to 650 cm−1. The euclidean distance was used to construct the dendrogram according to the absorption peaks.
The dendrogram divides the 14 samples into two separated clusters (Figure 2): cluster 1 (C1) has 2 species from the Pottiaceae (the control group), cluster 2 (C2) has 12 samples from the families Mielichhoferiaceae, Bryaceae, and Mniaceae. C2 is split into two second subclusters. Subcluster 1 (SC1) is composed of Rhodobryum roseum, Rhodobryum gigantenm, Pohlia elongate, and Bryum argenteum. Subcluster 2 (SC2) has five species from the family Minaceae. In SC1, the specie of genus Pohlia is clustered with B. argenteum (type of the genus Bryum). The result of the cluster analysis is inconsistent with the molecular results, which indicates that the species of genus Pohlia is closely related to the family Mniaceae. The phylogenetic relationship of family Pottiaceae is distant from Mielichhoferiaceae, Bryaceae, and Mniaceae. As expected, the two species of Pottiaceae are completely apart from the other species.
The results reflect the relative relationship between the 14 samples. Further study is needed to identify the internal relationships of the genera. PCA and one-dimensional DWT were adopted in our study.
PCA Analysis
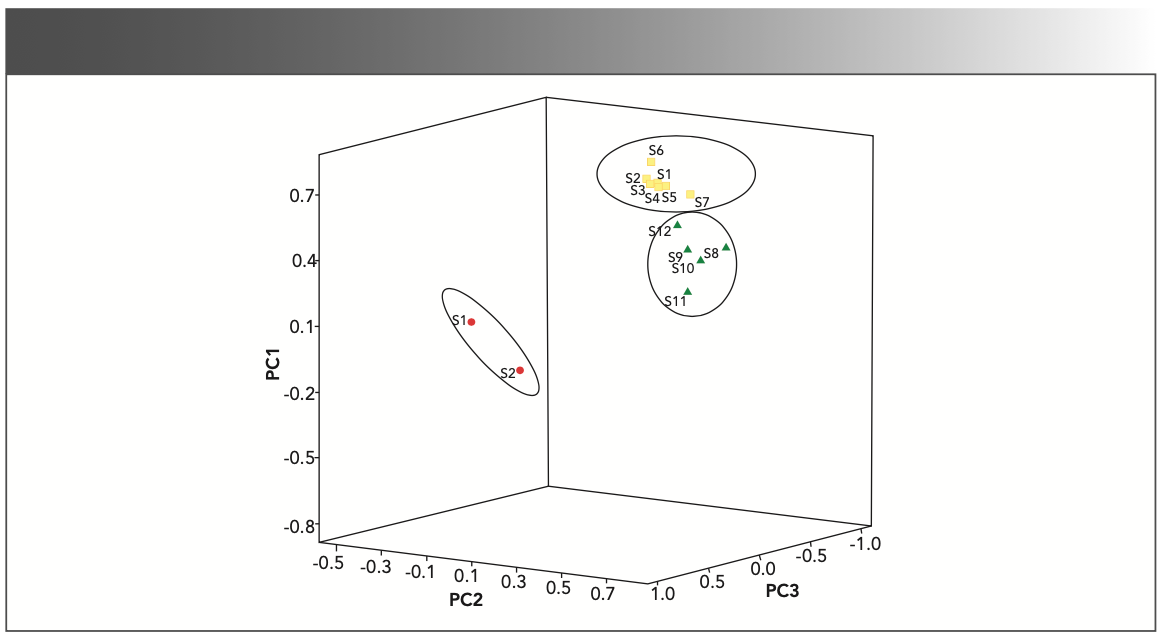
In this study, we used PCA as the second multivariate analysis technique. The data from the absorption peaks within the range of 4000 to 650 cm−1 in the FT-IR spectra were analyzed by PCA. Table III shows the variance that accounts for the first four PCs computed from the absorbance value of the characteristic peaks used in cluster analysis. The first three PCs summarized more variation in the data than any other PCs, accounting for more than 99.24% of the data variance. Figure 3 shows the PCA with overlaid clusters of the 14 samples based on the first three PCs. The overlaid clusters plot indicates that the species of families Bryaceae, Mielichhoferiaceae, Mniaceae, and Pottiaceae can be grouped into three separate sets (Figures 3 and 4). The studied species of Pohlia elogata, Bryum argenteum, and three species of genus Rhodobryum form one well-supported group (blue triangle plots, Figure 3). The Plagionium species exhibit a short-distance relationship with Mnium hornum and Trachycystis ussrriensis (green dots, Figure 3). The molecular evidence indicates that the species from genus Pohlia are closely related with the species from family Mniaceae (6). By contrast, the CLA and PCA data suggest that the species of genus Pohlia are more closely related to B. argenteum (type of genus Bryum and family Bryaceae). According to the scatter plots, the two samples from Pottiaceae are quite different from other samples. The 3D plots of FT-IR spectra of the 14 samples (Figure 4) analyzed by SPSS19.0 and the PCA with overlaid clusters analyzed by R ver. 3.6.1 (<www.r-project.org>) are in accordance with the result of CLA (Figure 2). The PCA results can be used to identify different families simply and accurately.
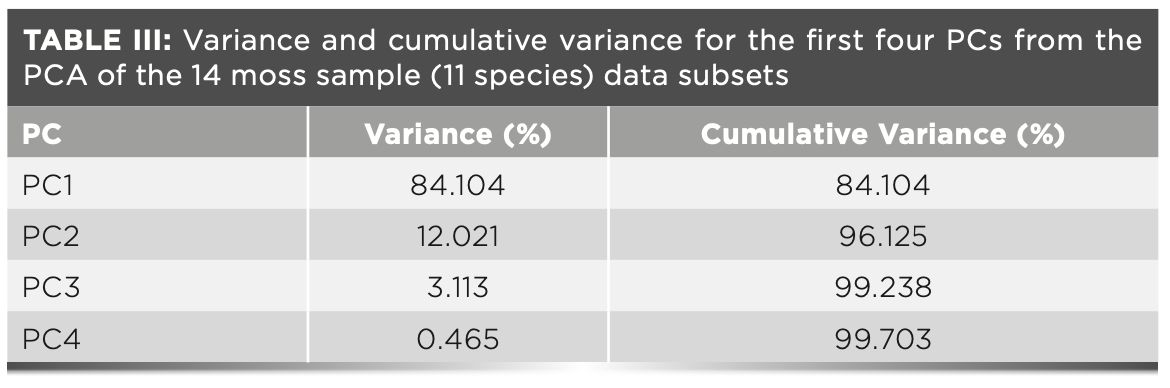
FIGURE 2: The dendrogram represents the relationship between the 14 moss samples clustered by hierarchical cluster analysis on the basis of FT-IR data.
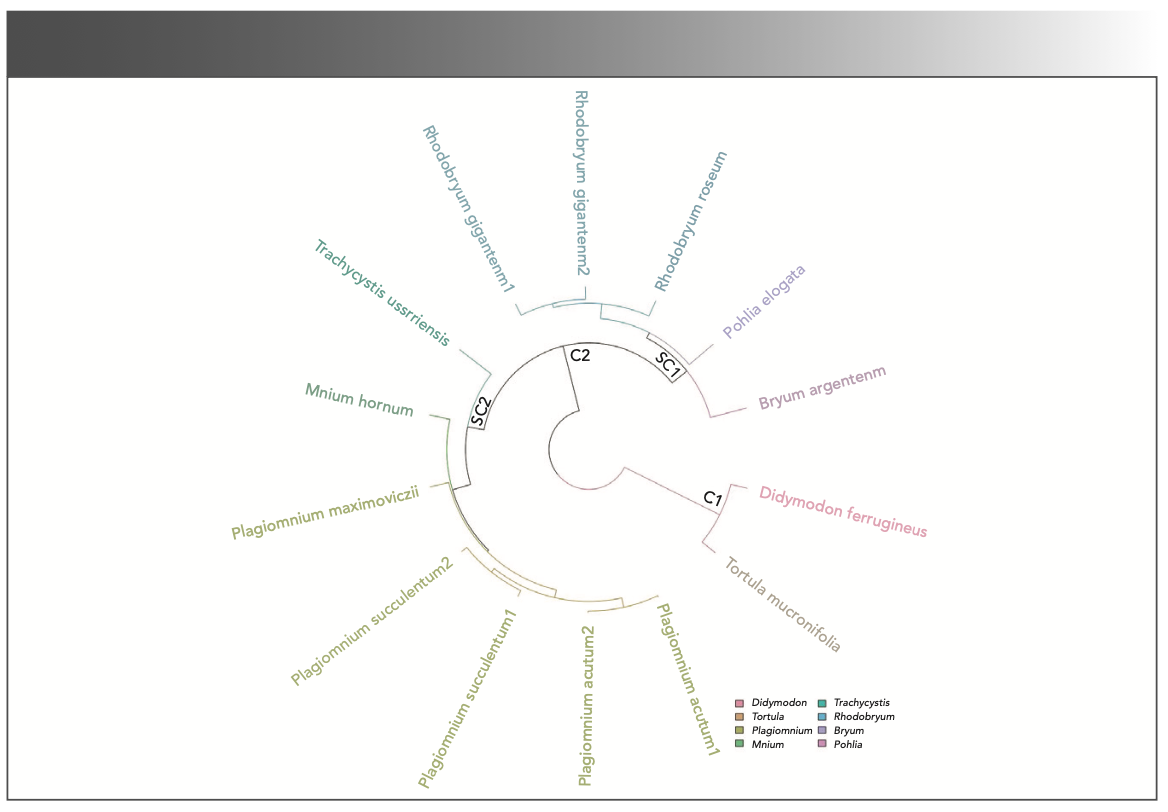
FIGURE 3: PCA with overlaid clusters of the 14 samples (11 species) of mosses.
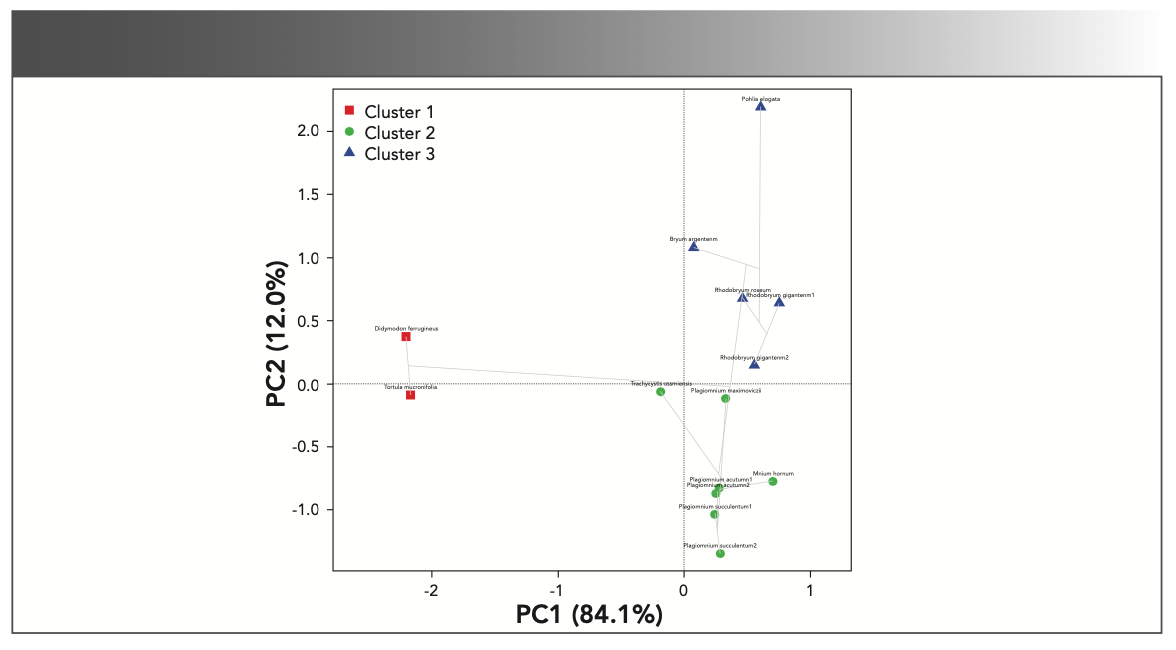
FIGURE 4: 3D plot of FT-IR spectra of the 14 samples (11 species) of mosses based on PCA.
Wavelet Analysis of FT-IR Spectral Data
Species that belong to the same genus contain similar chemical components, such as protein, carbohydrate, and plant hormones (36). FT-IR spectra of the same genus exhibit close peak absorbance and wavenumber values. In particular, the FT-IR absorptions of the species from different geographical locations are quite difficult to distinguish. Therefore, in our study, DWT was used to extract the FT-IR spectra features for further identification. The 1800 to 650 cm−1 region can provide higher characteristic molecular structural information on the spectra. The 1800 to 650 cm−1 fingerprint region contains more molecular structural information and has been used for DWT analysis.
The Classification of Species in Sect. Mniaceae, Sect. Mielichhoferiaceae, and Sect. Bryaceae
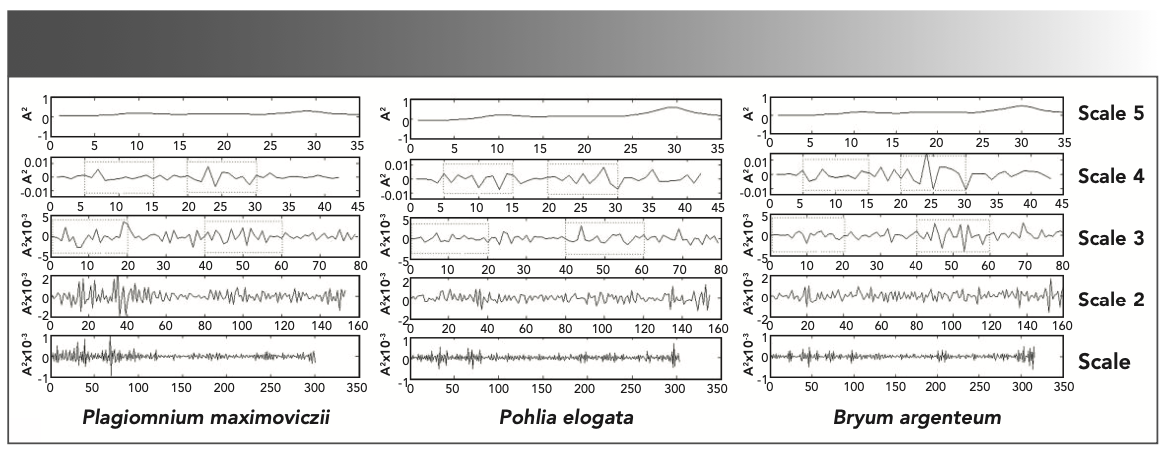
The genus Pohlia was traditionally included in the family Bryaceae. However, the phylogenetic position of genus Pohlia has changed in recent years. The phylogenetic analysis of random amplified polymorphic DNA (RAPD) makers showed that the genus Pohlia is more closely related to the genera of the family Mniaceae than Bryaceae (37). According to the CLA and PCA results, the species of genus Pohlia is more closely related to B. argenteum type species of Bryaceae, whereas the species of genus Pohlia has a distant relationship with the species of Mniaceae (Figures 2–4). We classified the ambiguous species of mosses based on their different chemical composition, which yields detailed information on different species. The PCA and CLA results can be considered as a piece of evidence on the phylogenetic status of genus Pohlia. To further identify the species of different genera, DWT was used to clarify with the FT-IR spectra. Plagiomnium maximoviczii, Pohlia elogata, and Bryum argenteum were chosen to represent Sect. Mniaceae, Sect. Pohlia, and Sect. Bryum. Scale 1–5 presents the detailed information after decomposition (Figure 5). The five scales were compared, and the DWT coefficients of Plagiomnium maximoviczii, Pohlia elogata, and Bryum argenteum were found to have great difference from one another in scale 2–4. Different species of the close relatives can be identified by DWT.
FIGURE 5: Results of the multi-resolution decomposition for FT-IR spectra with DWT of Plagiomnium maximoviczii, Pohlia elogata, and Bryum argenteum.
The Classification of Species in Sect. Plagiomnium
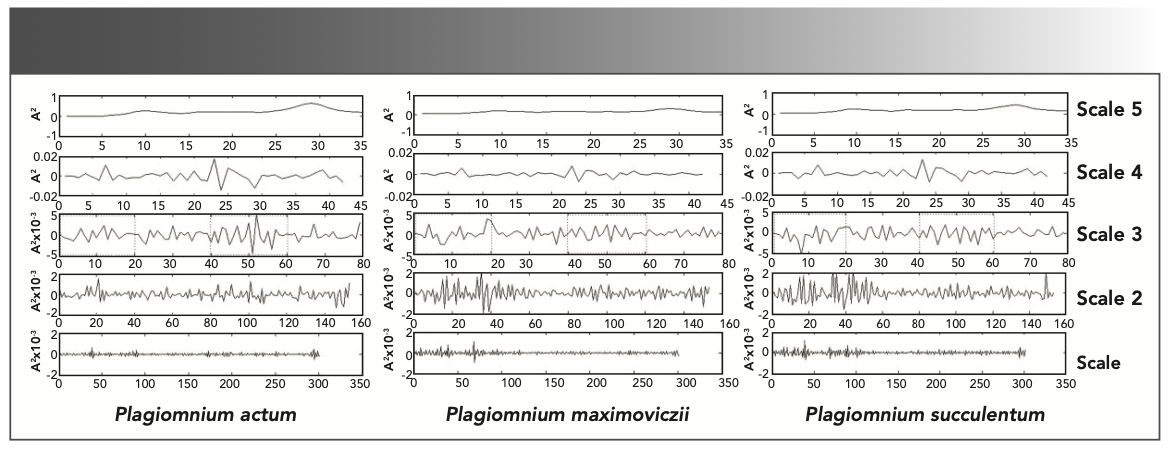
The FT-IR spectra form different species of the same genus have very close absorbance values at the same wavenumber. The DWT was used to enhance the differences between the species of different genera to achieve satisfactory results. So we use this method to the study of species in the same genus (Plagiomnium). Figure 6 shows the DWT coefficients of complete stool of P. actum, P. maximoviczii, and P. succulentum. We make 5-level wavelet decomposition to deal with the FT-IR spectra data. Scale 1-5 (Figure 6) shows detailed information after decomposition. The DWT coefficients can effectively affect the information features of spectra (35). After comparing the five scales, scales 2–4 are sensitive to the change of absorption frequency in the spectra. As the DWT coefficients of P. actum, P. maximoviczii, and P. succulentum have great difference, especially in scale 3 (Figure 6), different species of the same genus can be distinguished accurately.
FIGURE 6: Results of the multi-resolution decomposition for the FT-IR spectra with DWT of three species of genus Plagiomnium (P. actum, P. maximoviczii, and P. succulentum).
Comparison of the Different Samples of Plagiomnium actum
The two samples (P. actum-1 and P. actum- 2) were collected from different areas (Table I). The chemical components of the two samples of P. actum did not have much difference. The FT-IR spectra of the two samples were difficult to differentiate.
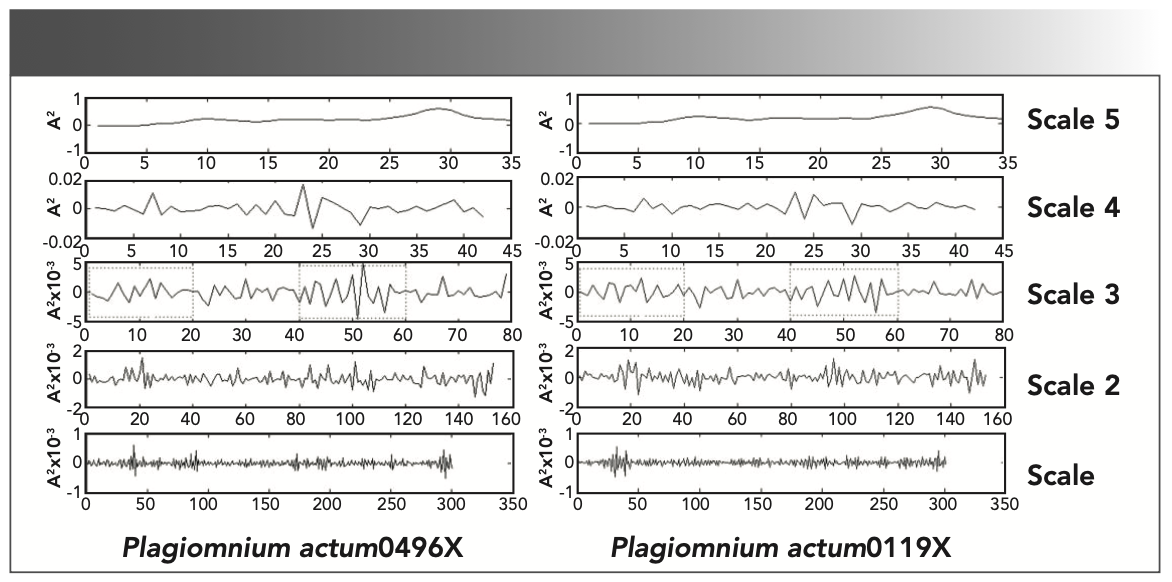
One-dimensional DWT was applied to decompose the FT-IR spectral data of P. actum-1 and P. actum-2 into different frequency bands. The vibration signals were decomposed up to five levels using Daubechies 4 mother wavelet. The DWT coefficients effectively reflected the features of the spectra (Figure 7). Scale 1 contains a high noise component, which is unsuitable in analyzing differences between the same species. Determining the differences in Scale 4, 5 were also difficult. Scale 2, 3 presents the difference between the three samples (Figure 7). Therefore, the decomposition levels 2, 3 in the DWT domain were chosen as a variable characteristic extraction region because it showed intraspecific variation of Plagiomnium actum. The results indicated that DWT can be used to extract the features of the FT-IR absorptions of different samples of the same species.
FIGURE 7: Results of the multi-resolution decomposition for FT-IR spectra with DWT of the two samples of Plagiomnium actum (Plagiomnium actum 0496X, Plagiomnium actum 0119X).
Conclusion
In recent years, the phylogenetic relationship of families Mielichhoferiaceae, Bryaceae, and Mniaceae has remained unclear. The traditional circumscriptions are based mainly on peristome characters, making classification of the taxa difficult. The results of the present study show that FT-IR spectroscopy in combination with PCA and cluster analyses can be used to differentiate the genera of Mielichhoferiaceae, Bryaceae, and Mniaceae. Recent studies based on molecular data indicate that genus Pohlia belongs to Mniaceae (38). In our study, we found that genus Pohlia is more closely related to Bryaceae based on the results of tests using chemical methods to determine the chemical composition. The PCA results show that the Pohlia species are not closely related to the species of Mniaceae. DWT are used to extract the features and enhance the differences between the species from different genera whose FT-IR spectra are similar. The species of Plagiomnium are successfully identified by FT-IR spectroscopy method combined with DWT. Results of the FT-IR spectroscopy combined with cluster analyses and PCA can also be used to identify different genera and families of mosses. FT-IR spectroscopy method combined with DWT is suitable for differentiating different species of mosses from controversial groups. The results show the possibility of using optical methods, such as FT-IR method, in differentiating the genera and species of mosses. FT-IR combined with chemometric methods for the identification of mosses is a rapid and efficient technique that could enable routine laboratories to facilitate identification of mosses.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (31660052, 42271045) and the Science and Technology Project of Hebei Provincial Department of Education (ZD2021035).
References
(1) Crosby, M. R.; Magill, R. E.; Allen, B.; et al. A Checklist of the Moss. Missouri Botanical Garden, St. Louis. 1999. www.mobot.org/MOBOT/tropicos/most/checklist.shtml
(2) Crandall-Stotler, B.J.; Bartholomew-Began, S.E. Morphology of Mosses (Phylum Bryophyta). Flora of North America 2007, 27, 3–13.
(3) Guerra, J.; Jiménez-Martínez, J.F.; Cano, M.J.; Jiménez-Fernández, J.A. A Contribution to the Phylogenetic study of Mielichhoferiaceae-Mniaceae (Bryophyta) Based on Molecular Sequence Data. Nova Hedwigia 2011, 93 (1), 47–56. DOI: 10.1127/0029-5035/2011/0093-0047
(4) Cox, C.J.; Hedderson, T.A.J. Phylogenetic Relationships Among the Ciliate Arthrodontous Mosses: Evidence from Chloroplast and Nuclear DNA Sequences. Plant. Syst. Evol. 1999, 215 (1-4), 119–139. DOI: 10.1007/BF00984651
(5) Cox, C.J.; Hedderson, T.A.J. Phylogenetic Relationships Within the Moss Family Bryaceae Based on Chloroplast DNA Evidence. J. Bryol. 2003, 25 (1), 31–40. DOI: 10.1179/037366803125002635
(6) Cox, C.J.; Goffinet, B.; Shaw, A.J.; Boles, S.B. Phylogenetic Relationships among the Mosses Based on Heterogeneous Bayesian Analysis of Multiple Genes from Multiple Genomic Compartments. Syst. Bot. 2004, 29 (2), 234–250. DOI: 10.1600/036364404774195458
(7) Wang C.Y.; Zhao, J.C. Phylogeny of Ptychostomum (Bryaceae, Musci) Inferred from Sequences of Nuclear Ribosomal DNA Internal Transcribed Spacer (ITS) and chloroplast rps4. J. Sys. Evol. 2009, 47, 311–320. DOI: 10.1111/j.1759-6831.2009.00033.x
(8) Asakawa, Y.; Ludwiczuk, A.; Nagashima, F. Phytochemical and Biological Studies of Bryophytes. Phytochem. 2013, 91, 52–80. DOI: 10.1016/j.phytochem.2012.04.012
(9) Cheng, C.G.; Liu, J.; Cao, W.Q.; Zheng, R.; Wang, H.; Zhang, C. Classification of Two Species of Bidens Based on Discrete Stationary Wavelet Transform Extraction of FTIR Spectra Combined with Probability Neural Network. Vib. Spectrosc. 2010, 54 (1), 50–55. DOI: 10.1016/j.vibspec.2010.06.004
(10) Wang, X; Chen, X.; Qi, Z.; XLiu, X.; WLi, W.; Wang, S. A Study of Ganoderma lucidum Spores by FTIR Microspectroscopy, Spectrochim. Acta, Part A 2012, 91, 285–289, DOI: 10.1016/j.saa.2012.02.004.
(11) Mariey, L.; Signolle, J.P.; Amiel, C.; Travert, J. Discrimination, Classification, Identification of Microorganisms Using FTIR Spectroscopy and Chemometrics. Vib. Spectrosc. 2001, 26 (2), 151–159. DOI: 10.1016/S0924-2031(01)00113-8.
(12) Achten, E.; Schütz, D.; Fischer, M.: Fauhl-Hassek, C.; Horn, B. Classification of Grain Maize (Zea mays L.) from Different Geographical Origins with FTIR Spectroscopy—a Suitable Analytical Tool for Feed Authentication? Food Anal. Methods 2019, 6, 26. DOI: 10.1007/s12161-019-01558-9
(13) Cebi, N.; Zeki-Durak, M.; Toker, O.S.; Sagdic, O.; Arici, M. An Evaluation of Fourier Transforms Infrared Spectroscopy Method for the Classification and Discrimination of Bovine, Porcine, and Fish Gelatins. Food Chem. 2016, 190, 1109–1115. DOI: 10.1016/j.foodchem.2015.06.065
(14) Wu, X.H.; Zhu, J.; Wu, B. Discrimination of Tea Varieties Using FTIR Spectroscopy and Allied Gustafson-Kessel Clustering. Computers and Electronics in Agriculture. 2018, 147, 64–69. DOI: 10.1016/j.compag.2018.02.014
(15) Naumann, D; Helm, D.; Labischinski, H. Microbiological Characterizations by FT-IR Spectroscopy. Nature 1991, 351 (6321), 81–82. DOI: 10.1038/351081a0
(16) D. Naumann, D. Infrared Spectroscopy in Microbiology, in Encyclopedia of Analytical Chemistry, Meyers, R. A. (Ed.) John Wiley & Sons, 2000, pp. 192–231.
(17) De Luca, M.; Terouzi, W.; Ioele, G.; Kzaiber, F.; Oussama, A.; Oliverio, F.; Tauler, R.; Ragno, G. Derivative FTIR Spectroscopy for Cluster Analysis and Classification of Morocco Olive Oils. Food Chem. 2011, 124 (3), 1113–1118, DOI: 10.1016/j.foodchem.2010.07.010.
(18) Cheng, C.G.; Tian, Y.M.; Zhang, C.J. A Novel Recognition Method Between Semen Cuscutae and Japanese Dodder Seed Based on FTIR-Continuous-Wavelet Feature Extraction and Artificial Neural Network Classification Method. Acta Chim Sin. 2008, 66 (7), 793–798.
(19) Cheng, C.G.; Tian, Y.M.; Jin, W.Y. A Study on the Early Detection of Colon Cancer Using the Methods of Wavelet Feature Extraction and SVM classifications of FTIR. Spectroscopy 2008, 22 (5), 397–404.
(20) Naumann, A.; Heine, G.; Rauber, R. Efficient Discrimination of Oat and Pea Roots by Cluster Analysis of Fourier Transform Infrared (FTIR) Spectra. Field. Crops. Res. 2010, 119 (1), 78–84. DOI: 10.1016/j.fcr.2010.06.017
(21) Jackson, M.; Mantsch, H.H. in Encyclopedia of Analytical Chemistry, Meyers, R. A., Ed. Wiley: Chichester, 2000, pp. 131–156.
(22) Poulli, K.I.; Mousdis , G.A.; Georgiou, C.A. Classification of Edible and Lampante Virgin Olive Oil Based on Synchronous Fluorescence and Total Luminescence Spectroscopy. Anal. Chim. Acta 2005, 542 (2), 151–156. DOI: 10.1016/j.aca.2005.03.061
(23) Daubechies, I. The Wavelet Transform, Time-Frequency Localization and Signal Analysis. IEEE Transactions on Information Theory 1990, 369 (5), 961–1005, DOI: 10.1109/18.57199.
(24) Lu, H.F.; Cheng, C.G.; Tang, X.; Hu, Z.H. FTIR Spectrum of Hypericum and Triadenum with Reference to Their Identification. Acta. Bot. Sin. 2004, 46 (4), 401–406.
(25) Marchant, B.P. Time–Frequency Analysis for Biosystems Engineering. Biosyst. Eng. 2003, 85 (3), 261–281. DOI: 10.1016/S1537-5110(03)00063-1.
(26) Subasi, A. Medical Decision Support System for Diagnosis of Neuromuscular Disorders using DWT and Fuzzy Support Vector Machines. Comput. Biol. Med. 2012, 42 (8), 806–815. DOI: 10.1016/j.compbiomed.2012.06.004.
(27) Salzer, R.; Steiner, G.; Mantsch, H.; et al. Infrared and Raman Imaging of Biological and Biomimetic Samples. Fresenius J. Anal. Chem. 2000, 366, 712–726. DOI: 10.1007/s002160051565
(28) Helm, D.; Labischinski, H.; Schallehn, G.; Naumann, D. Classification and Identification of Bacteria by Fourier-Transform Infrared Spectroscopy. J. Gen. Microbiol. 1991, 137 (1), 69–79. DOI: 10.1099/00221287-137-1-69
(29) Helm, D.; Harald Labischinski, H.; Dieter Naumann, D. Elaboration of a Procedure for Identification of Bacteria using Fourier-Transform IR Spectral Libraries: A Stepwise Correlation Approach. J. Microbiol. Meth. 1991, 14 (2), 124–127. DOI: 10.1016/0167-7012(91)90042-O
(30) Yu, P.Q.; Agric, J. Applications of Hierarchical Cluster Analysis (CLA) and Principal Component Analysis (PCA) in Feed Structure and Feed Molecular Chemistry Research, Using Synchrotron-Based Fourier Transform Infrared (FTIR) Microspectroscopy. J. Food. Chem. 2005, 53 (18), 7115–7127. DOI: 10.1021/jf050959b
(31) Kemsley, E.K.; Belton, P.S.; McCann, M.C.; Ttofis, S.; Wilson, R.H.; Delgadillo, I. Spectroscopic Method for the Authentication of Vegetable Matter. Food Control 1994, 5 (4), 241–243. DOI: 10.1016/0956-7135(94)90023-X.
(32) Defernez, M.; Kemsley, E. K.; Wilson, R. H. Use of Infrared Spectroscopy and Chemometrics for the Authentication of Fruit Purees. J. Agric. Food Chem. 1995, 43, 109–113.
(33) Sockalingum, G.D.; Bouhedja, W.; Pina, P, et al. FT-IR Spectroscopy as an Emerging Method for Rapid Characterization of Microorganisms. Cell. Mol. Bio. 1998, 44 (1), 261–269.
(34) Yang, J.W.; You, X.G.; Tang, Y.Y.; Fang, B. A Watermarking Scheme Based on Discrete Non-Separable Wavelet Transform. Pattern Recognition and Image Analysis 2005, 3522, 427–434.
(35) Hu, T.; Jin, W.Y.; Cheng, C.G. Classification of Five Kinds of Moss Plants with the Use of Fourier Transform Infrared Spectroscopy and Chemometrics. Spectroscopy 2011, 25 (6), 271–285.
(36) Asakawa, Y.; Ludwiczuk, A.; Nagashima, F. Phytochemical and Biological Studies of Bryophytes. Phytochemistry 2013, 91, 52–80, DOI: 10.1016/j.phytochem.2012.04.012.
(37) W. Q. Li, Master degree dissertation, Hebei Normal University, China. 51–55 (2006).
(38) Frey W.; Stech, M. Bryophytes and Seedless Vascular Plants. 3: I–IX, in Syllabus of Plant Families, Frey, W. Ed.; Gebr. Borntraeger Verlagsbuchhandlung, 2009, pp. 191–197.
Zhen Cao, Zhenjie Wang, and Dongmei Xu are with the College of Industry and Technology, in Shijiazhuang, China. De Gao is with the College of Life Science, Hebei Normal University, in Shijiazhuang, China. Yongying Liu is with Jiaozuo Normal College, in Jiaozuo, China. Peng Xu is with the Department of Mathematics and Statistics at Eastern Michigan University, in Ypsilanti, Michigan, USA. Direct correspondence to: de.gao@hebtu.edu.cn and wzjie1965@sina.com ●

Best of the Week: AI and IoT for Pollution Monitoring, High Speed Laser MS
April 25th 2025Top articles published this week include a preview of our upcoming content series for National Space Day, a news story about air quality monitoring, and an announcement from Metrohm about their new Midwest office.
LIBS Illuminates the Hidden Health Risks of Indoor Welding and Soldering
April 23rd 2025A new dual-spectroscopy approach reveals real-time pollution threats in indoor workspaces. Chinese researchers have pioneered the use of laser-induced breakdown spectroscopy (LIBS) and aerosol mass spectrometry to uncover and monitor harmful heavy metal and dust emissions from soldering and welding in real-time. These complementary tools offer a fast, accurate means to evaluate air quality threats in industrial and indoor environments—where people spend most of their time.
New AI Strategy for Mycotoxin Detection in Cereal Grains
April 21st 2025Researchers from Jiangsu University and Zhejiang University of Water Resources and Electric Power have developed a transfer learning approach that significantly enhances the accuracy and adaptability of NIR spectroscopy models for detecting mycotoxins in cereals.
AI Shakes Up Spectroscopy as New Tools Reveal the Secret Life of Molecules
April 14th 2025A leading-edge review led by researchers at Oak Ridge National Laboratory and MIT explores how artificial intelligence is revolutionizing the study of molecular vibrations and phonon dynamics. From infrared and Raman spectroscopy to neutron and X-ray scattering, AI is transforming how scientists interpret vibrational spectra and predict material behaviors.