Lanthanide Series Investigations with TSI LIBS Desktop Analyzer
Application Notebook
The lanthanide series is a series of metallic elements, with atomic numbers 58 through 71, which are - in order of increasing atomic number - cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, and lutetium.
The lanthanide series is a series of metallic elements, with atomic numbers 58 through 71, which are — in order of increasing atomic number — cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, and lutetium. They have numerous commercial uses based on their individual chemical, optical, and nuclear properties. Examples of commercial use include use in control rods in nuclear reactors (gadolinium, dysprosium), as colors in glasses and enamels (praseodymium, neodymium, cerium), and as constituents in laser medium and solid state devices (neodymium and terbium).

Figure 1: Neodymium measurements with TSI LIBS Desktop Analyzer.
Lanthanides are often measured with spectroscopic means such as atomic absorption spectroscopy (AAS) and inductively coupled plasma (ICP) optical emission spectroscopy (OES). These methods involve acid digestion of the matrix prior to analysis. Direct analysis methods such as XRF can be problematic as the L series transitions used to analyze these elements often overlap the K series fluorescence from transition elements.

Figure 2: Europium measurements with TSI LIBS Desktop Analyzer.
The TSI® LIBS Desktop Analyzer Solution
The TSI LIBS Desktop Analyzer uses a technique known as laser induced breakdown spectroscopy (LIBS) to directly determine the elemental concentrations of lanthanides in materials. The examples below of lanthanide determination in graphite matrix illustrate that determination of dilute concentrations (low ppm) in many matrices is possible.
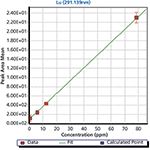
Figure 3: Lutetium measurements with TSI LIBS Desktop Analyzer.
TSI Inc.
500 Cardigan Rd, Shoreview, MN 55126
tel. (800) 874-2811
Website: www.tsi.com

Thermo Fisher Scientists Highlight the Latest Advances in Process Monitoring with Raman Spectroscopy
April 1st 2025In this exclusive Spectroscopy interview, John Richmond and Tom Dearing of Thermo Fisher Scientific discuss the company’s Raman technology and the latest trends for process monitoring across various applications.
A Seamless Trace Elemental Analysis Prescription for Quality Pharmaceuticals
March 31st 2025Quality assurance and quality control (QA/QC) are essential in pharmaceutical manufacturing to ensure compliance with standards like United States Pharmacopoeia <232> and ICH Q3D, as well as FDA regulations. Reliable and user-friendly testing solutions help QA/QC labs deliver precise trace elemental analyses while meeting throughput demands and data security requirements.