Mapping Protein Conformational Stability Using Automated CD
Application Notebook
This study describes a new automated technique for measuring protein conformation by CD spectroscopy and how changes can be followed in different pH environments.
David Gregson and Lindsay Cole, Applied Photophysics, Ltd.
This study describes a new automated technique for measuring protein conformation by CD spectroscopy and how changes can be followed in different pH environments. The study confirms the robustness of the technique and superb sample-to-sample reproducibility. The improvements in productivity enable experiments to be undertaken that were previously thought to be too time consuming or laborious.
Correct protein function is critically dependent upon conformation — if a protein is not correctly folded, it will not execute its function or worse, it may be toxic. Circular dichroism spectroscopy (CD) is an exquisitely sensitive probe of protein conformation, but it is often perceived as a technique limited to more specialized applications. This is to some extent because, up until now, CD spectrometers have remained resolutely manual in operation which has now been overcome by new automation technology.
Experimental Conditions
Solutions of BSA, sodium phosphate, and sodium citrate were used to prepare a series of buffered protein samples from pH 2.2 to pH 8. The samples were prepared directly in a 96-well plate and each protein solution was paired with its buffer. Buffers and samples were transferred to the spectrometer using a fixed-probe x, y, z robot, and CD and absorption spectra measured simultaneously and in duplicate. Each complete measurement, including washing, took 7.5 min to perform.
Results
Each protein CD and absorption spectrum was baseline-corrected using its paired buffer (Figure 1).
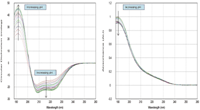
Figure 1: Buffer-subtracted CD and absorption spectra for BSA (260 nm ⥠λ ⥠188 nm) from pH 2.2 to pH 8.
Plotting the raw data as a contour map gives an immediate semi-quantitative feel for the stability of the protein. Using quantitative methods, such as the global fitting of a model to the data, make it clear even to the untrained eye where regions of conformational stability exist (Figure 2). A two-state model was used to describe the data and although it is known that this model may be inadequate to describe the behavior of serum albumins completely (1), it is good enough to show the potential of the method. End-point spectra for high- and low-pH were calculated as was the pKa of the transition between them.

Figure 2: Calculated conformational stability map (CD contours). The loss of structure below pH 4.2 indicates clearly that the protein conformation changes at low pH.
Conclusion
The automated sample preparation and measurement of CD and absorption spectra have been shown to be reliable, giving quantitative results with minimum effort using robust global analysis techniques. Excellent reproducibility and sample economy together with the ability to carry out an unattended measurement at a rate of 192 samples per day considerably improves the productivity of CD measurements. These improvements offer the opportunity to study relative protein stabilities with ease, for example, in protein biotherapeutic development and clone selection procedures.
References
(1) D.C. Carter and J.X. Ho, "Structure of Serum Albumin" Advances in Protein Chemistry, Vol. 45, Lipoproteins, Apolipoproteins and Lipases, Academic Press 1994, Ed. Verne Schumaker. ISBN: 0-12-034245-6. p. 153–203.

Applied Photophysics Ltd.
201-205 Kingston Road, Leatherhead, Surrey, United Kingdom
tel. +44 1372 386537; fax +44 1372 386477
Email: sales@photophysics.com; Website: www.photophysics.com

Enhancing Trace Element Analysis with Inductively Coupled Plasma Mass Spectrometry
May 15th 2025Elemental analysis is crucial in a wide variety of applications from detecting toxic elements within the environment, to ensuring drinking water is safe for human consumption, to food product safety. ICP-MS—able to measure an atom’s mass—offers low detection limits in the range of parts per trillion (ppt), making it a widely used method that can detect toxic elements well below regulatory limits. This paper expands upon how new ICP-MS technology can meet the challenges associated with heightened demands for element analysis and the hurdles laboratories face when analyzing high-matrix samples.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)














