Organic Nitrogen Compounds X: Nitro Groups, An Explosive Proposition
We review one final organic nitrogen functional group, representing explosive compounds. This is the nitro group, which requires a different set of interpretation rules.
Our final look at the infrared spectra of organic nitrogen functional groups will be of the nitro, or NO2, group. This group has two strong and characteristic infrared features, making it easy to identify. However, attaching a NO2 group to a molecule scrambles its electronic structure, which is why these compounds are explosive, and why nitro groups make some of the infrared interpretation rules we learned irrelevant.
Over the several years that I have been writing these articles, there have been large sections of material devoted to the spectroscopy of functional groups that contain specific chemical bonds. When I began discussing organics containing nitrogen, I stated that the stretching and bending of N-H bonds would be important in determining whether or not there was nitrogen present in a molecule (1). We subsequently used N-H stretching and bending peaks to analyze the spectra of amines (2,3), amides (4,5), imides (6), and urethanes (7).
The nitro group is going to be the odd man out in this discussion. This functional group consists of a nitrogen atom with no hydrogens, but with two oxygens and a carbon attached, as seen in Figure 1.
Figure 1: The structural framework of the nitro functional group. The dashed lines denote nitrogen-oxygen “bonds and a half.”
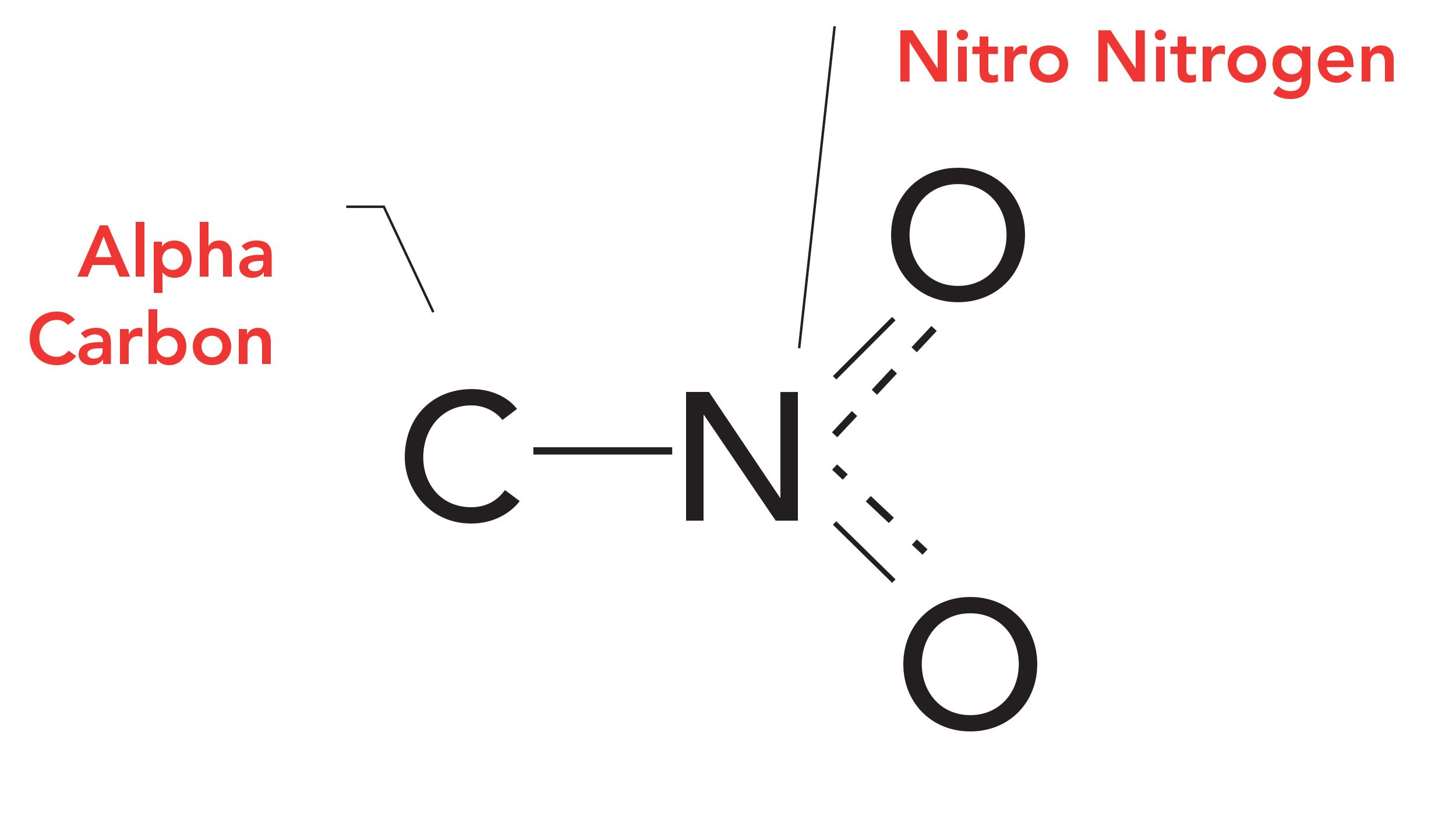
Note that the nitrogen in the NO2 group is called the nitro nitrogen, and that the carbon atom singly bonded to the nitro nitrogen is called the alpha carbon. Depending on whether the alpha carbon is saturated, or part of an aromatic ring, nitro molecules can be divided into saturated and aromatic nitro compounds.
The chemical bonding of the NO2 group is unusual. Normally, oxygen atoms form two chemical bonds (8). However, there is also a C-N bond in the nitro group, as seen in Figure 1. Given that nitrogen normally forms three bonds (8), how do we apportion the electrons in a nitro group to keep these compounds from falling apart?
There are three bonding electrons shared between the two oxygens in the nitro group, giving what are essentially two “bonds and a half,” as seen in Figure 1. The dashed lines in Figure 1 represent the half bonds. These NO bonds are similar to the carboxylate C-O bonds and a half discussed in a previous column (9). Neither oxygen atom has its full complement of two full chemical bonds, making the nitro groups unstable. Saturated nitro compounds such as nitroalkanes, otherwise known as rocket fuel (8), are rarely analyzed by infrared spectroscopy because they are liable to detonate during analysis. We will not study them further.
However, nitro groups attached to benzene rings can be relatively stable, assuming not too many nitro groups are attached. The chemical structure of trinitrotoluene, a commonly used explosive known as TNT, is shown in Figure 2.
Figure 2: The chemical structure of trinitrotoluene (TNT).
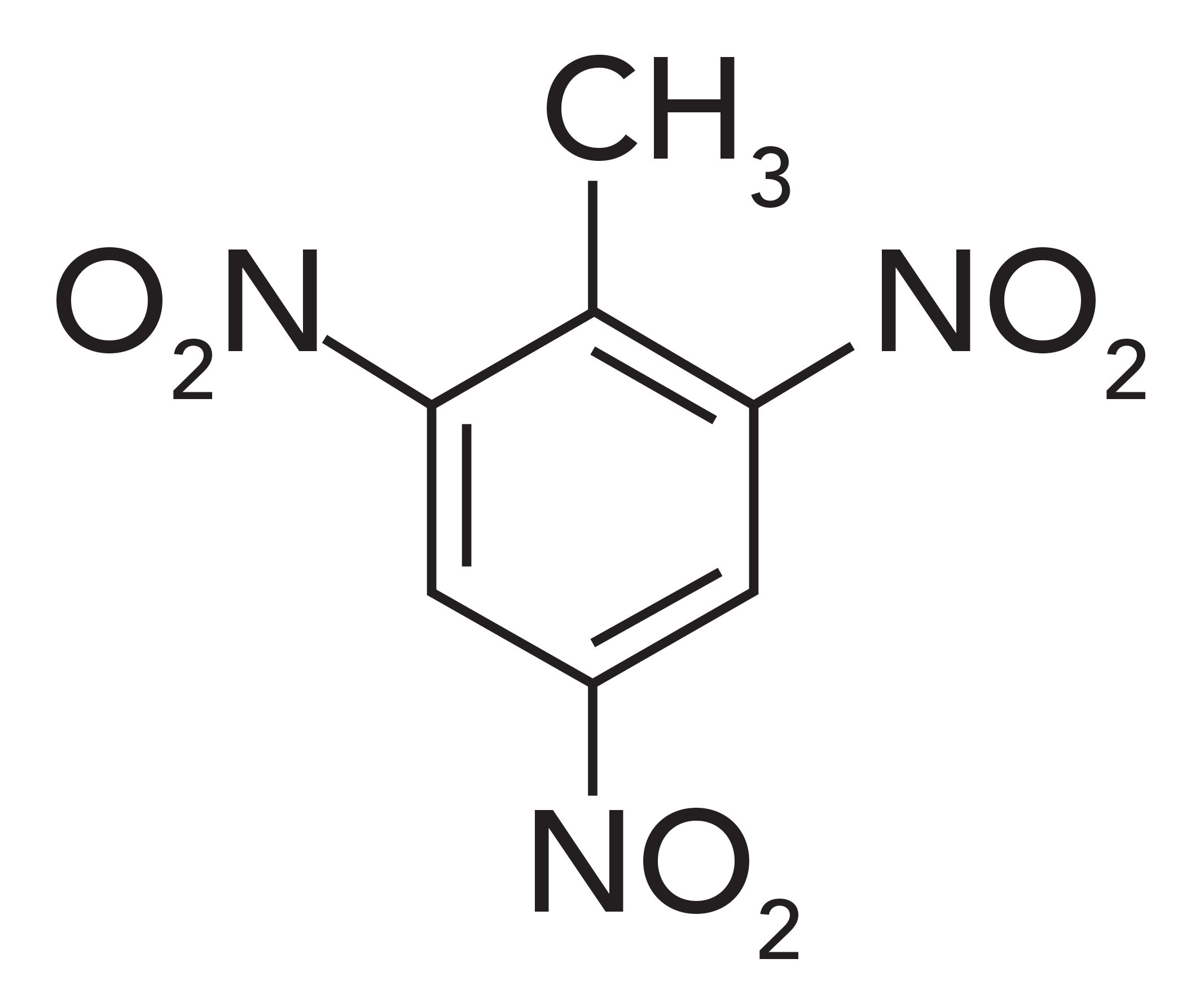
The presence of the three nitro groups destabilizes the benzene ring, resulting in TNT’s explosive properties. Di-nitrotoluene and mono-nitrotoluene are stable. We will restrict our discussion to non-explosive aromatic nitro compounds.
The Infrared Spectroscopy of the Nitro Functional Group
The infrared spectrum of an aromatic nitro compound, meta-nitrotoluene, is seen in Figure 3. Recall that highly polar bonds have intense infrared features due to the large change in dipole moment with respect to bond length, dµ/dx, during a vibration (10). Oxygen is more electronegative than nitrogen; therefore, the N-O bonds in the nitro group are relatively polar, and as a result, their asymmetric and symmetric stretching peaks are unusually large. The details of these vibrations are shown in Figure 4.
Figure 3: The infrared spectrum of meta-nitrotoluene.
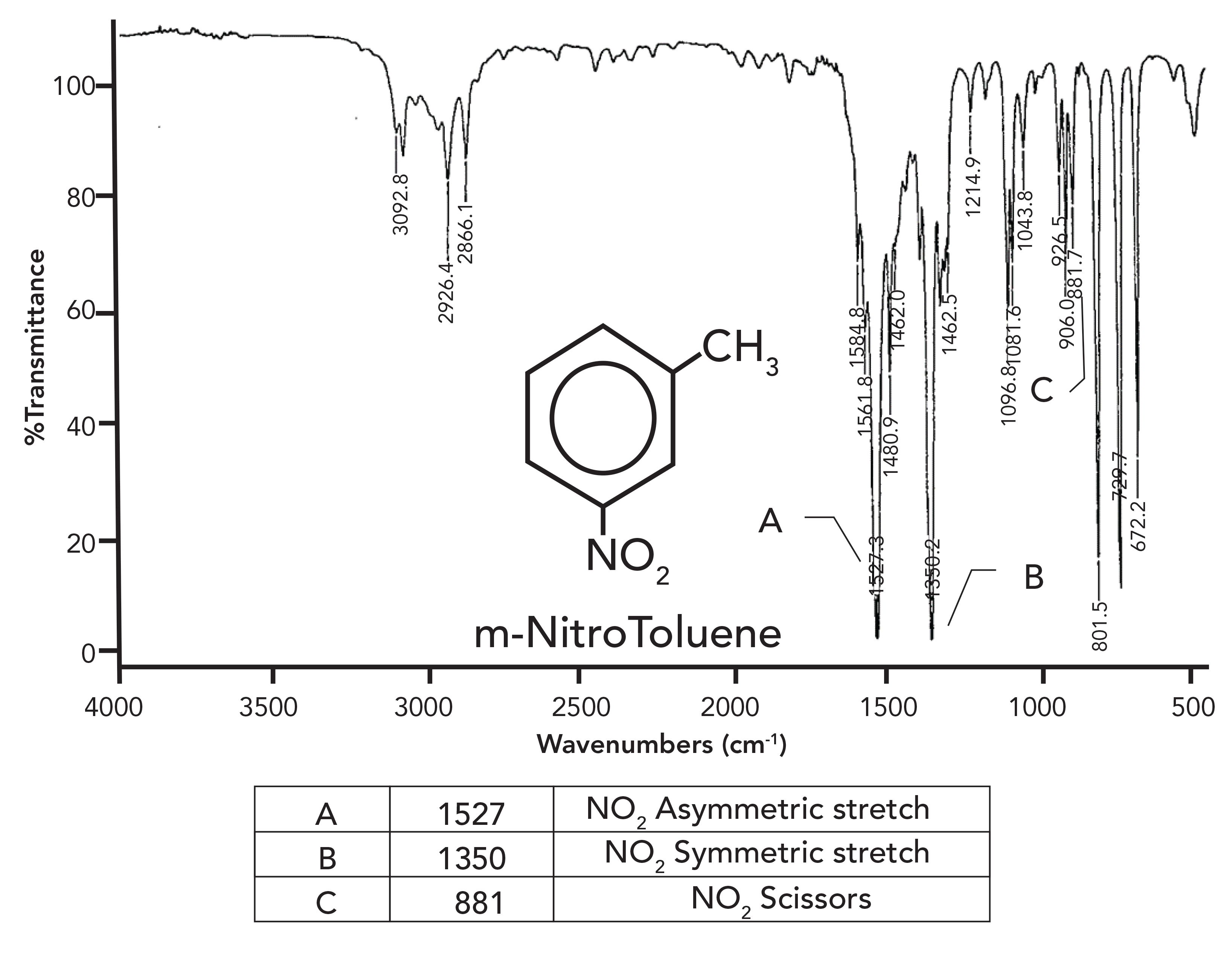
Figure 4: The asymmetric and symmetric stretches of the NO2 functional group.
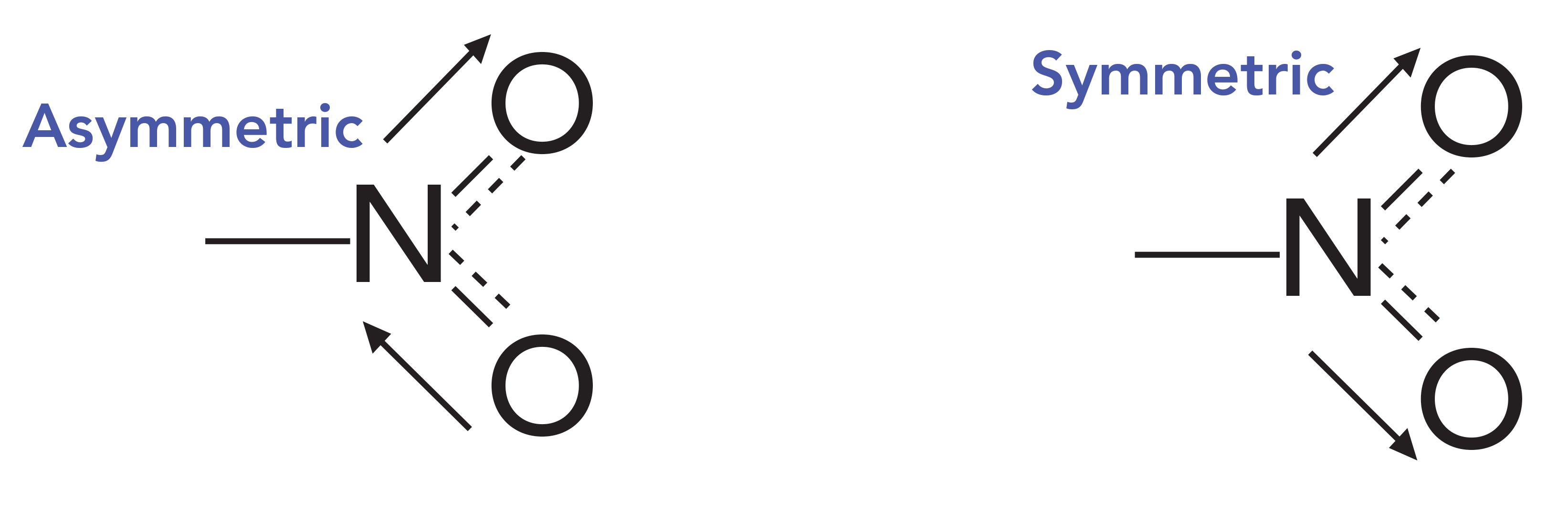
The asymmetric NO2 stretch typically falls from 1550 to 1500 cm-1, and is seen in Figure 3 labeled A at 1527 cm-1 (assume all peak positions are in cm-1 units, even if not labeled as such). The symmetric stretch is seen in Figure 3 labeled B at 1350 cm-1, and, in general, this peak appears from 1390 cm-1 to 1330 cm-1. Note how peaks A and B in Figure 3 are the two most intense peaks in the spectrum, and they stick down like eye teeth in the middle of the spectrum. The combination of a pair of intense peaks in these wavenumber ranges is unique, making the presence of a nitro group in a sample easy to spot.
The nitro group also exhibits a scissors bending vibration, similar to that of the methylene group (11). This gives rise to a medium intensity peak from 890 cm-1 to 835 cm-1. It can be seen in Figure 3 labeled C at 881 cm-1.
The good news about the nitro group is that it has two strong infrared bands that are easy to spot. The bad news is that whenever the nitro group is attached to a benzene ring, it makes it difficult to determine the substitution pattern on the benzene ring. Recall that the benzene ring out-of-plane C-H bend band, in combination with the presence or absence of the aromatic ring-bending band at 690 cm-1, can be used to determine the substitution pattern on a benzene ring (12). The presence of a nitro group makes it difficult to apply these rules. This is caused by the unique electronic structure of the nitro group, and how it interacts electronically with the benzene ring. Suffice it to say, one may need to use an analytical technique other than infrared spectroscopy to determine the substitution pattern on nitro-substituted aromatic rings.
Note that the structure of meta-nitrotoluene does contain a methyl group. We have learned that the diagnostic pattern for a methyl group includes saturated carbon asymmetric and symmetric stretches near 2962 cm-1 and 2872 cm-1, and the umbrella mode at 1375 cm-1 (13,14). Note in Figure 3 that the saturated C-H stretches fall at 2926 cm-1 and 2866 cm-1. Under normal circumstances, we would interpret these two peaks as the asymmetric and symmetric stretches of methylene groups, because they have peaks at 2926 cm-1 and 2855 cm-1 (14). In this case, that interpretation is wrong, because the nitro groups scramble the electronic structure of the molecule, throwing off these peak positions. If you notice the nitro peaks first, it will give you a heads up that the saturated C-H stretches may be troublesome. Normally we can depend on the methyl group umbrella mode to indicate that the saturated C-H stretches could be an issue. However, in this case, the intense nitro symmetric stretching peak at 1350 cm-1 is sitting on top of it. Unfortunately, then there is nothing in this spectrum clearly showing the presence of the methyl group. As stated above, the nitro group throws off some of the interpretation rules we have learned.
The diagnostic pattern for the presence of a nitro group in a sample then is a pair of intense peaks at about 1550 cm-1 and 1350 cm-1, along with the scissor peak around 850 cm-1. Table I summarizes the group wavenumbers for the nitro group.
Conclusions
The nitro group consists of a nitrogen atom with two oxygens and one carbon attached. The two nitrogen-oxygen bonds are “bonds and half” that destabilize nitro compounds, making their analysis an explosive proposition. The large dipole moments for the NO bonds give two strong peaks around 1550 cm-1 and 1350 cm-1 as a result of the asymmetric and symmetric stretching of the NO2 functional group. This is an unusual pattern, and is easy to spot. There is also a scissoring peak around 850 cm-1. Nitro groups tend to scramble the electronic structure of molecules, making the interpretation of their spectra problematic.
References
- B.C. Smith, Spectroscopy 34(1), 10–15 (2019).
- B.C. Smith, Spectroscopy, 34(3), 22–25 (2019).
- B.C. Smith, Spectroscopy, 34(5), 22–26 (2019).
- B.C. Smith, Spectroscopy, 34(11), 30–33 (2019).
- B.C. Smith, Spectroscopy 35(1), 10–15 (2020).
- B.C. Smith, Spectroscopy 35(3), 26–30 (2020).
- B.C. Smith, Spectroscopy 35(5), 17–21 (2020).
- A. Streitwieser and C. Heathcock, Organic Chemistry (Macmillan, New York, New York, 1976).
- B.C. Smith, Spectroscopy 34(5),20–23(2018).
- B.C. Smith, Spectroscopy 30(1), 16–23 (2015).
- B.C. Smith,Spectroscopy 30(7), 26–31, 48 (2015).
- B.C. Smith,Spectroscopy 31(5), 36–39 (2016).
- B.C. Smith, Spectroscopy, 30(4), 18–23 (2015).
- B.C. Smith, Infrared Spectral Interpretation: A Systematic Approach (CRC Press, Boca Raton, Florida, 1999).
QUIZ SECTION
Your Next Infrared Spectral Interpretation Challenge
Using everything you have learned in this and previous columns, determine the functional groups present in the Figure i spectrum and try to determine the chemical structure of this compound. Remember that inclusion of a peak position in the table does not necessarily mean that it will be useful in the structure determination.
Figure i:The mid-infrared spectrum of a liquid measured using the capillary thin film method.
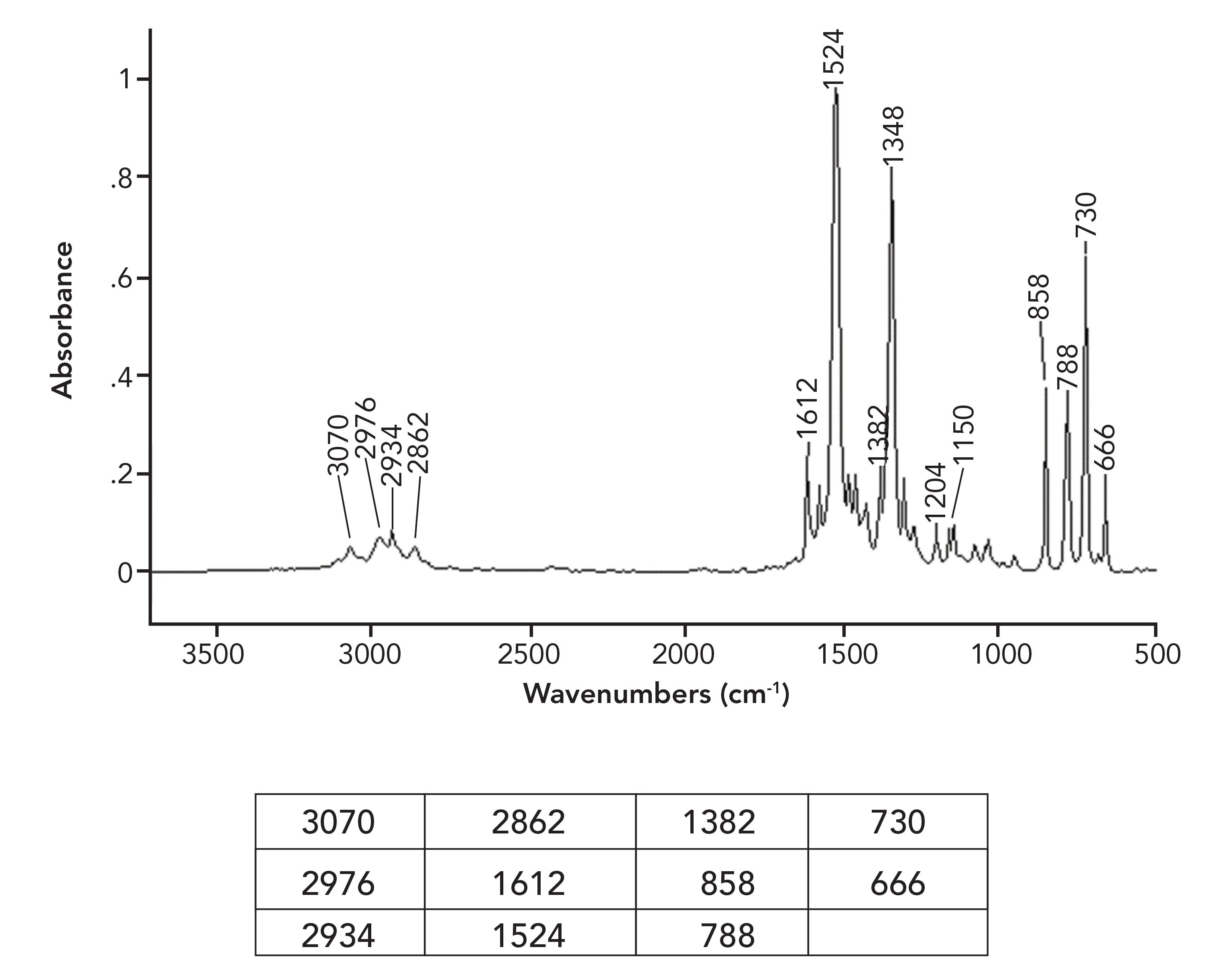
Because this is a particularly tricky problem, answer these questions about the spectrum in this order to guide you. In all cases, be sure to justify your answer.
1. Is there a nitro group present?
2. If so, is there a benzene ring present?
3. Are there any other functional groups present?

Brian C. Smith, PhD, is the founder and CEO of Big Sur Scientific, a maker of portable mid-infrared cannabis analyzers. He has over 30 years experience as an industrial infrared spectroscopist, has published numerous peer-reviewed papers, and has written three books on spectroscopy. As a trainer, he has helped thousands of people around the world improve their infrared analyses. In addition to writing for Spectroscopy, Dr. Smith writes a regular column for its sister publication Cannabis Science and Technology and sits on its editorial board. He earned his PhD in physical chemistry from Dartmouth College. He can be reached at: SpectroscopyEdit@MMHGroup.com

AI Shakes Up Spectroscopy as New Tools Reveal the Secret Life of Molecules
April 14th 2025A leading-edge review led by researchers at Oak Ridge National Laboratory and MIT explores how artificial intelligence is revolutionizing the study of molecular vibrations and phonon dynamics. From infrared and Raman spectroscopy to neutron and X-ray scattering, AI is transforming how scientists interpret vibrational spectra and predict material behaviors.
Real-Time Battery Health Tracking Using Fiber-Optic Sensors
April 9th 2025A new study by researchers from Palo Alto Research Center (PARC, a Xerox Company) and LG Chem Power presents a novel method for real-time battery monitoring using embedded fiber-optic sensors. This approach enhances state-of-charge (SOC) and state-of-health (SOH) estimations, potentially improving the efficiency and lifespan of lithium-ion batteries in electric vehicles (xEVs).
New Study Provides Insights into Chiral Smectic Phases
March 31st 2025Researchers from the Institute of Nuclear Physics Polish Academy of Sciences have unveiled new insights into the molecular arrangement of the 7HH6 compound’s smectic phases using X-ray diffraction (XRD) and infrared (IR) spectroscopy.