Grating Corrected Laser Stabilization: A Case Study in Pharmaceutical Raw Material Identification
Special Issues
The authors present a novel technique for obtaining very high stability and reproducibility of a Raman spectrum, using grating corrected laser stabilization. An externally stabilized laser with a grating spectrometer provides exceptional quantum efficiency in the entire dynamic range. These components then are used to build a library of pharmaceutical raw materials and tested on samples of unknown material.
Raman spectroscopy is becoming a more commonly used technique for chemical analysis. Raman analysis is nondestructive, requires very limited sample preparation, and allows for the analysis of sample volumes in the microliter range. Also, Raman techniques can be used to acquire data through vial walls, pill pack windows, and bags — packaging forms that are frequently used in the biomedical and pharmaceutical industries. A major barrier to the acceptance of Raman in these industries is the cost of high-resolution Raman systems, often upward of $40,000.
In recent years, low resolution Raman spectroscopy (LRRS) has gained increased attention and acceptance in the analytical laboratory as an affordable, convenient, and robust alternative to cumbersome wet chemical analysis or expensive FT-Raman and FT-IR spectrometers. LRRS systems relinquish resolution in favor of emphasizing basic identifying spectral features, or fingerprints, that often are distinct and clearly separated. High-resolution Raman systems perform very well, but might be unnecessary for most simple spectral identification applications.
The introduction of the silicon charged-coupled device (CCD) detector was the first technological development to enable LRRS. The CCD sensor has replaced the more light-sensitive single-element detectors such as the photomultiplier tube and avalanche photodiode. Depending upon the coating applied to the CCD sensor, it can detect light in the 200-1200 nm range. Integrated into an optical bench, the CCD can allow for resolution as fine as 4 cm-1. A back-thinned CCD detector, which is more light-sensitive than a standard CCD, has been integrated into Raman spectrometers allowing for 6 cm-1 resolution, increased quantum efficiency, and high signal-to-noise ratios (S/N). Advances in these devices have improved wavelength stability and increased S/N, both crucial factors in building libraries and performing unambiguous spectral identification of raw materials.
Another development that has contributed to the acceptance of LRRS in identification applications is the availability of low-cost laser diodes used as excitation sources. Unlike single-mode lasers that demand careful control of the laser line, diode lasers often have multiple modes with the instrument utilizing one of the available modes to excite the sample. While this advantage translates into a reduction of the price tag of the overall system, it introduces one drawback, the "mode-hopping" phenomenon, which can significantly degrade the repeatability of a spectral measurement.
Portable, high-resolution spectrometers used in Raman applications are able to display the spectral shifts associated with mode-hopping occurrences. Spectral shifts of as much as 8-10 cm-1 are sometimes observed among consecutive spectra, significantly reducing the transferability of a calibration model or a library from one instrument to another. A variety of techniques have been used to correct this problem, including fiber and volume Bragg gratings and distributed feedback lasers (DFBs).
This article demonstrates a technique to obtain very high wavelength stability and reproducibility of a Raman spectrum using an inexpensive alternative to other single-mode laser options called grating-corrected laser stabilization. Proof-of-concept of this laser is first shown with a comparison in pixel drift between an off-the-shelf multimode laser and the grating-corrected laser. Finally, a specific Raman application is shown for pharmaceutical raw material fingerprinting and comparison with unknown samples.
Methods and Materials
Two separate Raman spectrometers were used for this study. An Ocean Optics (Dunedin, FL) QE65000 Spectrometer (configured for the appropriate measurement range) was used for the first phase of the study. The QE65000 has a back-thinned detector that can be cooled to -10 °C with the onboard TE-cooler to reduce dark noise. The reduction of noise and dark signal allows integration times of up to 10 min for low-light level applications.
Two integrated R-3000HR instruments from Raman Systems, Inc. (Austin, TX) were used for the cross-comparison of pharmaceutical material libraries. The R-3000HR integrated system contains both a laser and an Ocean Optics HR2000 Spectrometer that can provide resolution of 6 cm-1.
The off-the-shelf multimode laser is an undisclosed laser of relatively poor quality. The grating-corrected wavelength isolation laser, developed by Raman Systems, has a patent pending and is fully integrated into the Raman Systems R-3000HR system. Both lasers have approximately the same power output of 500 mW.
The samples studied consisted of simple organics, and raw and active pharmaceutical ingredients. They were contained in standard, clear borosilicate scintillation vials with no additional preparation applied. Most samples were purchased from Sigma-Aldrich (Milwaukee, WI) or were obtained from a major pharmaceutical company. The Raman probes used with the Raman R-3000HR were standard lab probes provided by Raman Systems. The probes used with the QE65000 were standard probes provided by InPhotonics (Norwood, MA). Data was acquired using the prepackaged spectrometer system software with subsequent library analysis performed using Thermo Galactic's (Salem, NH) Spectral ID software.
Results and Discussion
Proof-of-Concept.
The grating- corrected wavelength isolation approach should ensure reproducibility of Raman spectra on the
x
-axis, thereby facilitating easy transfer of calibration models independent of spectral acquisition systems. The set of experiments described below show the results from measurements made with a grating-corrected multimode laser. We compare these results with the observed variation in the wavelength (8-10 cm
-1
) of a noncorrected multimode 785-nm diode laser.
Spectra for mixed xylenes, which are very rich in spectral features, were measured using the back-thinned spectrometer with the multimode laser over a 12-h period. Spectral data was acquired every 10 min to establish the x-axis drift. The results are shown in Figure 1.
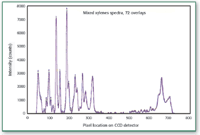
Figure 1. Spectra of mixed xylenes collected over a 12-h period, one spectrum every 10 min, total of 72 spectra. Spectra collected using a back-thinned, TE-cooled CCD-array detector and a low-performance multimode laser.
This same experiment was performed on the integrated Raman system containing the grating-corrected laser on m-xylene over 12 h with spectral acquisition every 12 min. The results for the integrated system are shown in Figure 2.
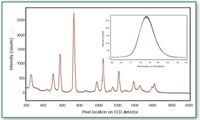
Figure 2. m-Xylene spectra collected over a 12-h period, one spectrum every 12 min, total of 60 spectra. The reproducibility of the peaks is better than a single pixel of the CCD. The inset plot is a closer look at the strongest peak of the spectrum 1000 cm-1 peak of m-xylene.
As demonstrated in the pixel shift comparison between the two figures, the relatively inexpensive solution to correcting poor-quality multimode lasers provides exceptional pixel stability. This stability is required to make Raman instrument-independent libraries for fingerprinting pharmaceutical samples. Also, because grating-corrected lasers are a less-expensive alternative to single-mode lasers, LRRS are attractive for the pharmaceutical QA/QC laboratories.
Case Study. An internal study was performed to illustrate the importance of reproducibility of spectra along the x axis. Here a raw pharmaceutical material identification application within the pharmaceutical industry was performed. The point to note is not so much the unambiguous identification of the unknown, but the transfer of the calibrated library from one instrument to another, facilitated by the reproducibility of the selected laser wavelengths.
In this study, 12 pharmaceutical raw materials were obtained, five of which were clear liquids and seven of which were white powders. The study was conducted on the two higher-resolution instruments with both integrated systems containing the new grating-corrected lasers.
Spectra for all of the materials were recorded on the first Raman instrument (with the grating-corrected laser), and a library then was created, based upon the fingerprint peaks identified by the acquisition software. After the complete library was acquired, spectra of two "unknown" samples, one clear liquid and one white powder, were then recorded on the other instrument (also containing the grating-corrected laser) and tested with the library.
Identification was unambiguous. The unknowns were identified easily with the application of the grating-corrected isolation laser with the maximum off-shift of peaks from one instrument to the other of 2 cm-1. Figure 3 shows the overlay of library sample 5 and unknown 1 (collected on the different instruments).
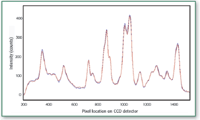
Figure 3. Overlay of Library Spectrum 5 and Unknown 1 collected on different Raman instruments.
Table I lists the relevant peaks identified in the library software. Sample 5 was recorded on the first Raman instrument and compared with the peaks identified in the sample unknown 1, recorded on the second Raman instrument.
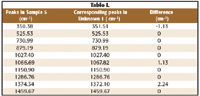
Table I.
The same comparison was made with unknown 2. The library immediately detected that unknown 2 was sample 7 in the library (sample will remain unnamed due to proprietary issues). Table II lists the peaks identified for sample 7 recorded on the first instrument and then compared with the peaks identified in the unknown 2, recorded on the second instrument.
Cross-correlation of libraries in different instruments definitely is possible when using the grating-corrected laser. The largest shift is 2.24 cm-1, which still is much smaller than the standard multimode laser shift of up to 8-10 cm-1.
Conclusions
When comparing with library spectra and the subsequent transfer of such models across instruments, one of the most important requirements for unambiguous identification of peaks is the reproducibility of the spectral features. This study has demonstrated that by using a grating-corrected isolation of the excitation wavelength, reproducibility within an average of 1 cm
-1
can be obtained. Such grating-corrected, low-cost multimode diode lasers help make Raman a preferred method for quick and non-destructive testing. This methodology has proved extremely reproducible and provides definitive pharmaceutical material identification.
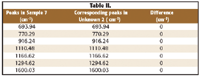
Table II.
Sameer Londhe is director of business development for Raman Systems (Austin, TX). Tina Bacon and Yvette Mattley are senior scientists, and Jay Thomason is marketing communications manager, for Ocean Optics (Dunedin, FL). E-mail: Jay.Thomason@OceanOptics.com.

AI-Powered SERS Spectroscopy Breakthrough Boosts Safety of Medicinal Food Products
April 16th 2025A new deep learning-enhanced spectroscopic platform—SERSome—developed by researchers in China and Finland, identifies medicinal and edible homologs (MEHs) with 98% accuracy. This innovation could revolutionize safety and quality control in the growing MEH market.
New Raman Spectroscopy Method Enhances Real-Time Monitoring Across Fermentation Processes
April 15th 2025Researchers at Delft University of Technology have developed a novel method using single compound spectra to enhance the transferability and accuracy of Raman spectroscopy models for real-time fermentation monitoring.