High performance Mass Spectroscopy Begins with High-Performance Separations
Special Issues
This article discusses the role of recent LC developments in th quest for greater sensitivity, more complete sample characterization, and greater productivity.

Liquid chromatography–mass spectrometry (LC–MS) labs in many industries need to detect lower levels of analytes, characterize complex samples more thoroughly, and perform analyses faster and at lower cost. While there are a number of ways to address these challenges, chromatography plays a key role in the quest for greater sensitivity, more complete sample characterization, and greater productivity. A pivotal development has been the availability of LC columns with smaller particles. Short versions of these columns can dramatically decrease analysis time while simultaneously producing sharper peaks for increased sensitivity. Longer versions can increase resolution for complex samples. Another development has been microfluidic LC chips that improve sensitivity, resolution, throughput, and ease of use. This article will discuss the contribution of these LC developments for enhanced productivity and streamlined analyses for LC–MS labs.
Columns for Faster Analyses
Many LC–MS labs are challenged to increase sample throughput or to perform more analyses with fewer instruments. One way to achieve faster analyses without sacrificing resolution is to switch to columns of the same bonded phase with smaller, sub-2-μm particles. Relative to columns with 5-μm and 3.5-μm particles, columns with sub-2-μm particles exhibit decreased plate height, which means more theoretical plates for a given column length. Because columns with smaller particles are more efficient, it is possible to use shorter columns and still keep the same resolution. Because analysis time is proportional to column length, shorter columns translate directly to shorter analysis times and increased throughput.
As an added benefit, columns with smaller particles maintain efficiency at higher flow rates; the van Deemter curves flatten out at higher linear velocities. Because analysis time is inversely proportional to flow rate, higher flow rates offer more potential time savings. With mass spectrometers, it is important to choose a column with an internal diameter that keeps the flow rate within the specified operating range for the LC–MS system. Fortunately, new ion source technology (such as a commercially available multimode ion source) can accommodate high flow rates in both electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI) modes.
Labs need not sacrifice sensitivity in their pursuit of faster analyses. Another reason that columns with sub-2-μm particles are attractive is that they provide sharper peaks, which results in improved sensitivity. As a final benefit, shorter columns with faster analyses use less mobile phase, saving the costs of solvent purchase and disposal.
Analysis of Drugs of Abuse Demonstrates Speed and Sensitivity
Figure 1 shows a typical fast analysis on a column with sub-2-μm particles. The figure shows elution in less than 1.5 min of four drugs of abuse and their internal standards on a 50-mm column with 1.8-μm particles. The drugs were analyzed by multiple reaction monitoring (MRM) using a triple quadrupole MS. Because the method required both quantifier and qualifier ions for each of the four analytes and their internal standards, 16 MRM transitions were monitored simultaneously. Despite splitting the signal 16 ways, the system was sufficiently sensitive to easily quantitate the drugs at the cutoff levels specified by the U.S. Substance Abuse and Mental Health Services Administration (SAMHSA). The LC column contributed to both the speed and the sensitivity of the analysis.
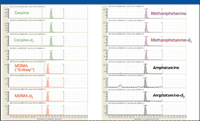
Figure 1: Extracted ion chromatograms from the LCâMS-MS analysis of four drugs of abuse in oral fluid demonstrate elution in less than 1.5 min. Column: 50 mm X 2.1 mm, 1.8-μm Agilent ZORBAX Rapid Resolution High Throughput (RRHT) SB-C18; instrumentation: Agilent model 1200 Series Rapid Resolution LC system with model 6410 triple quadrupole LCâMS detection; flow rate: 0.5 mL/min.
Getting the Best Performance from Columns with Sub-2-μm Particles
To take full advantage of columns with sub-2-μm particles, one needs to pay careful attention to a number of factors in the rest of the LC–MS system. These considerations include dead volumes, data acquisition rate, and backpressure.
To optimize performance for columns with small particles, it is critical that the liquid chromatograph have an appropriate delay volume. Some newer liquid chromatographs are designed for use with these columns, and they will work without modifications. Kits are available to update some older liquid chromatographs. For fast analyses with gradient operation, the volume between the pump and the head of the column should be appropriate so that the changing solvent composition reaches the column quickly. With existing liquid chromatographs, this might require use of a solvent mixer of lower volume and configuration of the sampler for lower delay volume. Some advanced liquid chromatographs are optimized for fast analyses; they have no solvent mixer or damper, and they have electronic damping control.
To avoid postcolumn band broadening, it is also important to adjust dead volume between the exit of the column and the mass spectrometer. Because many MS systems also incorporate UV detection, it is helpful to have a choice of flow cells for UV detectors. This allows one to optimize either UV sensitivity (in case quantitation is done by UV and the MS is used only for identification) or overall peak dispersion (in case optimal resolution is required for both UV and MS).
It is also important to ensure that the data acquisition rate of the mass spectrometer keeps pace with the very narrow peaks that are eluted from the column. This issue can be addressed by using quadrupole MS systems in single ion monitoring mode, or by using triple quadrupoles in MRM mode. In addition, time-of-flight (TOF) mass spectrometers acquire spectra very quickly and are a good match for fast LC.
Back pressure is another consideration for LC analyses that use smaller-diameter particles. The system back pressure results from both the column and the capillary tubing that connects the components in the system. For the column, the back pressure is inversely proportional to the square of the particle size, so as the particles get smaller, the backpressure rises rapidly. This effect can be minimized by use of columns with an engineered particle size distribution with a very low level of fine particles. Back pressure also can be reduced by use of higher temperatures to reduce solvent viscosity. Fortunately, fast LC generally uses shorter columns, which also reduces the back pressure. In cases where the column must be longer to maintain peak capacity for complex samples, it is important that the LC system be compatible with both higher temperatures and back pressures.
Columns for Better Resolution
While many LC–MS labs focus on faster analyses, others have complex, multicomponent samples and need to achieve better separations. For labs that need to more completely characterize samples that contain hundreds of components, longer columns with sub-2-μm particles are a good solution. Because columns with sub-2-μm particles have more theoretical plates per unit length, they provide greater resolution than columns of the same length with larger particles.
Analysis of Ginseng Root Extract Demonstrates Chromatographic Resolution
The advantage of smaller particles is obvious from Figure 2, which compares analyses of a ginseng root extract on a traditional LC system and a column with larger particles versus an optimized LC system and a column with smaller particles. The optimized LC system was designed for low system volume and high system back pressure. It could handle the back pressure for the analysis easily with the 1.8-μm particles, which was about 520 bar.
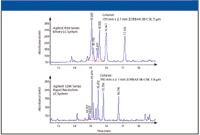
Figure 2: UV chromatograms from analysis of ginseng root extract show the resolution enhancement on a column with smaller particles. To maintain performance, such a column requires an optimized LC system.
The ginseng root extract shown in Figure 2 is a complex mixture of compounds that includes some medically active components. LC–MS-MS techniques have been used to elucidate structures of ginsenosides, the active components of ginseng root, and to quantify them (1). Figure 3 shows a spectrum from the analysis of ginseng root extract by TOF-MS. With their very fast spectral acquisition rates (on the order of 20–40 spectra/s), TOF systems are a good match for high-resolution chromatography with very narrow peaks. Even if the peaks are only one to two seconds wide, the clean TOF spectra permit identification of components that are partially coeluted. In the example in Figure 3, the spectral acquisition rate was fast enough that the TOF could alternate between two fragmentor voltages—a low fragmentor to generate molecular ions, and a high fragmentor to generate fragments for structural elucidation. The combination of high-resolution LC with TOF detection provides more complete sample characterization for complex samples with many narrow peaks.

Figure 3: TOF spectrum of ginsenoside Rb1 shows accurate mass measurement on the time scale of high-resolution LC. Masses are accurate to within +/â2.3 ppm. Column: 50 mm X 2.1 mm, 1.8-μm Agilent ZORBAX RRHT SB-C18; instrumentation: Agilent model 1200 Series Rapid Resolution LC with model 6210 time-of-flight LCâMS detection; flow rate: 0.5 mL/min.
Microfluidic LC Chips for Improved Nanoflow LC–MS
Microfluidic LC chips are another development that enables labs to achieve results faster and with improved sensitivity, resolution, and ease of use. Such devices are an alternative to conventional nanoflow chromatography with 75-μm (or smaller) columns. They can combine multiple chromatography steps on an integrated chip, which reduces troublesome connections and dead volumes. By delivering the full potential of 75-μm nanoflow column technology, the LC chip improves chromatographic resolution and sensitivity and enables better MS and MS-MS coverage of complex samples such as protein digests. Better coverage in turn leads to the identification of more sample components in a shorter length of time.
Conventional nanoflow LC–MS is very popular for ultratrace analysis because it combines both high-resolution chromatography and highly sensitive MS detection. However, the technique is a challenge to implement and maintain because of the large number of capillary tubing connections and the difficulty of keeping the system completely free of leaks, blockages, and excessive dead volumes.
A typical nanoflow LC–MS setup is comprised of an enrichment column to concentrate the sample, an analytical column, a valve to switch the flows between the columns, a nanospray emitter, and the capillaries and fittings to connect them. The development of microfluidic LC chips has enabled a tremendous reduction in the complexity of such a system. The majority of the plumbing connections can be integrated on a microfluidic chip that is smaller than a credit card, eliminating most potential trouble spots in the system.
Figure 4 shows a schematic of a microfluidic chip that integrates enrichment and analytical columns, nanospray emitter, fittings, and connection capillaries directly on a reusable polymer chip. The chip is fabricated from polyimide film, a material that is resistant to most solvents, tolerates a wide pH range, and is compatible with the analysis of many analytes, including peptides. The fabrication process uses UV laser ablation in combination with vacuum lamination of the polyimide film to create a multilayer microfluidic device (2). Open microchannels are packed with reversed-phase column materials to create high performance liquid chromatography (HPLC) columns. Metals are applied by thin film deposition onto the polymer film surfaces to produce the electrical contacts for electrospray.

Figure 4: Schematic of Agilent HPLC-Chip shows integration of enrichment column, analytical column, and nanospray emitter. Top view, analysis mode, with flow path in red.
The chip loads automatically into an interface between the LC system and the mass spectrometer. A specially designed microvalve in the interface handles high-pressure flow switching that enables alternating sample loading and sample analysis. The system is completely automated and more reliable than conventional nanospray, and is easier to set up and use.
Proteomics Example Shows Chip Advantages
Along with ease of use, microfluidic chips bring enhanced chromatographic performance, which is attributed to a number of factors. First, because integration of components on the chip eliminates most plumbing connections, dead volumes are reduced. Second, sample adsorption is minimized by the use of biocompatible polyimide and the elimination of troublesome connectors susceptible to sample adsorption. Third, because the electrospray emitter is integrated into the chip, postcolumn peak dispersion is negligible. Overall, the optimized design of the sample pathway minimizes sample loss and reduces dead volume. These enhancements significantly increase the number of identified peptides and proteins with the chip format.
Figure 5 compares an analysis of a mixture of 16 protein digests on a microfluidic chip and a conventional nanocolumn of the same dimensions. The figure demonstrates that the peaks are two to three times narrower with the chip system. Sharper peaks mean that the analytes are eluted in a smaller volume of solvent, which translates to better sensitivity for concentration-dependent detectors like MS. The increased chromatographic resolution also reduces the number of peptides that are eluted simultaneously, which reduces competing ionization and simplifies the mass spectra.
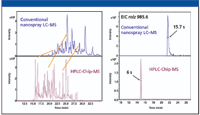
Figure 5: Comparison of chromatography between the Agilent HPLC-Chip and conventional nanoflow LCâMS shows better resolution with the HPLC-Chip. Analysis was a 30-min gradient separation of a mixture of 16 protein digests at varying concentrations. Detection: Agilent model 6330 ion trap LCâMS system; flow rate: 300 nL/min.
In some experiments, the primary concern is the number of proteins identified from a particular digest. Figure 6 shows the impact of the improved chromatographic performance of the microfluidic chip versus conventional nanoflow LC. The results show that the chip format produced a 32% increase in the number of unique identified peptides. This in turn led to a 15% increase in the number of identified proteins.
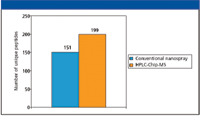
Figure 6: Comparison of peptide identifications between the Agilent HPLC-Chip and conventional nanoflow LCâMS demonstrates how the improved chromatography with the chip leads to identification of more peptides.
A recent study describes evaluation of the microfluidic chip for expression profiling and protein identification of rat plasma samples using both ion trap and TOF-MS (3). The authors found excellent reproducibility in retention time and intensity with the microfluidic chip.
The chip's potential for increased productivity and sensitivity is alleviating bottlenecks in a number of labs. "Using HPLC-Chip, we have more than doubled our productivity, analyzing four to six samples per hour instead of the one or two that we could do with classical nanoflow-LC, and all at increased sensitivity," said Dr. David Stapleton, research transfer facility manager, Bio21 Molecular Science and Biotechnology Institute, Melbourne, Australia.
Sensitive Chip-based Analysis of Small Molecules
Small-molecule LC–MS applications also have started to benefit from microfluidic chips, where advantages include easier analysis, improved chromatography, and better sensitivity. Figure 7 shows the use of a microfluidic chip for automated sample enrichment and nanoflow analysis of sulfadimethoxine in serum. The detection limit was 10 fg, which is extremely low and suggests the potential for sensitive determinations of similar analytes.
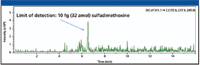
Figure 7: Sensitive LCâMS-MS analysis of sulfadimethoxine in serum used an Agilent HPLC-Chip for automated sample concentration and analysis and an Agilent model 6320 ion trap LCâMS system for detection.
Conclusion
In the quest for faster, more sensitive, and more complete MS analyses, LC plays a key role. Two key developments have been columns with smaller particles and microfluidic LC chips. Efficient columns with sub-2-μm particles — and LC systems that are optimized to support them — can be used for many applications. Shorter versions of these columns enable faster analyses, while longer versions exhibit improved resolution for more detailed analyses of complex samples. The commercial availability of microfluidic LC chips makes it easier to set up and use nanoflow LC–MS. The integration of multiple columns and the nanospray emitter on a single microfluidic chip reduces chromatographic dispersion and sample adsorption, and leads to better sensitivity and sample coverage. Microfluidic LC chips and columns with sub-2-μm particles make it possible to gain productivity, realize lower limits of detection, and identify more sample components. When researchers maintain the same stationary phase chemistry, it is easy to transfer methods from standard HPLC technology to these new technologies.
Georges L. Gauthier and Stefan Schuette are with Agilent Technologies, Waldbronn, Germany.
Christine Miller is with Agilent Technologies, Santa Clara, California.
References
(1) X. Wang, T. Sakuma, E. Asafu-Adjaye, and G.K. Shiu, Anal. Chem. 71, 1579–1584 (1999).
(2) H. Yin, K. Killeen, R. Brennen, D. Sobek, M. Werlich, and T. van de Goor, Anal. Chem. 77(2), 527–533 (2005).
(3) M.-H. Fortier, E. Bonneil, P. Goodley, and P. Thibault, Anal. Chem. 77(6), 1631–1640 (2005).

New Study Reveals Insights into Phenol’s Behavior in Ice
April 16th 2025A new study published in Spectrochimica Acta Part A by Dominik Heger and colleagues at Masaryk University reveals that phenol's photophysical properties change significantly when frozen, potentially enabling its breakdown by sunlight in icy environments.