Performance Characterization of ion Detectors in Harsh Environments
Special Issues
Recent developments in sample inlet systems, improved software, and the advent of special purpose mass spectrometers have improved the utility of MS instruments. here, the authors discuss this new utility.

For over 30 years, the mass spectrometer has been a trusted analytical tool in the laboratory. Today, mass spectrometers are used in such widely varying applications as food processing, semiconductor manufacturing, space exploration, forensics, drug discovery, energy exploration, and medical diagnostics. Mass spectrometers are even used as an early warning system for volcano eruptions and in homeland security and are playing a key role in the discovery of biomarkers for desease pathology.
Although other analytical approaches such as FT-IR, Raman, and UV–vis spectroscopy have been simpler to implement historically, they lack the specificity and sensitivity of mass spectrometry. Recent developments in sample inlet systems, improved software, and the advent of special purpose mass spectrometers have improved the utility of made instruments.
The typical mass spectrometer has three key components: The ionization source, which converts the unknown material into ions; the mass filter, which separates the resultant ions based upon the mass-to-charge ratio; the ion detector, which detects and amplifies the ion signal at each mass, which is then used to produce a mass spectrum by the instrument computer.
Applications in homeland security are now driving the development of field-portable instruments that can provide laboratory-grade analysis. To reduce the size, weight, and power consumption of a portable mass spectrometer, it is often necessary to deviate from the normal operating environment inside the vacuum system.
Designers of field-portable mass spectrometers face three challenges:
- Reduce the size
- Reduce the weight
- Reduce the power consumption
A quick assessment of most existing systems reveals that the vacuum system is the most significant contributor in all three categories.
For a mass spectrometer to operate, the unknown substance first must be ionized before it can be separated by mass and identified. All forms of mass separation require transporting the ions from the ionization source through the mass filter and onto the ion detector. Preventing multiple collisions with molecules within the spectrometer is essential for high sensitivity analysis. Therefore, the quality of the vacuum becomes an important consideration in designing portable instruments.
According to the kinetic theory of gases, the average distance an ion can travel without a collision with a gas molecule (mean free path) is determined by chamber pressure, the temperature, and the collision diameter of the ions (1). It is plain to see, therefore, that to prevent significant ion molecule collisions in portable instruments, the distance the ions must travel from ionization through the mass filtering process and to the detector must be kept as short as possible.
The Vacuum System
Conventional high vacuum pumps generally are not acceptable for portable instruments. Cryogenic vacuum pumps and oil diffusion pumps generally are not suitable because of weight and size, while ion pumps have a small pumping capacity.
Reducing the vacuum requirements of the mass spectrometer by reducing the ion flight length would simplify the pumping system significantly and reduce cost, weight, and power consumption.
Molecular drag pumps and low-cost oil-based roughing pumps can achieve vacuums as low as 5 × 10–4 Torr, while dry scroll pumps can achieve vacuum levels into the 10–2 Torr range.
Ion Sources
The most common method of ionization for field-portable instruments is electron ionization (EI). Filaments that operate near atmospheric pressure have been developed using yttrium coatings. In recent developments, some miniature systems now utilize electrical discharges to create the ions.
The Mass Filter
Typical matrix-assisted laser desorption ionization (MALDI) time-of-flight (TOF) mass spectrometers require an ion flight path length of 1–2 m. These instruments must operate in vacuums of 10–7 Torr or better.
Modern quadrupole based instruments typically operate at vacuum pressures in the high 10–6 Torr range because ions need to travel 35 cm or more before they reach the detector.
Most conventional ion traps and magnetic sector instruments require an operating vacuum of 10–5 Torr or better.
The key factor in reducing the vacuum requirements is to reduce the ion flight path. If it were possible to shrink the ion flight path length to less than 5 cm (approximately 2 in), then it should be possible to operate a system in the millitorr range. The Inficon XPR2 instrument (Inficon, Syracuse, New York) utilizes a 17-mm-long quadrupole mass filter.
Miniature Ion Trap-based instruments (Purdue University, Wes Lafayette, Indiana) fabricated from etched silicon substrates are reported to operate at or near atmospheric pressure. Although miniature quadrupoles and ion traps can operate at elevated pressures, their utility is very limited without the use of an electron multiplier to detect the ions.
The Ion Detector
A Faraday cup is an ion detector in its simplest form. Ions colliding with the metal cup deposit their charge which is detected by a high gain electrometer. The advantage of this type of ion detector is that it operates well at all vacuum levels. This type of detector does not incorporate any gain feature, however, and therefore seriously limits the sensitivity of the mass spectrometer.
Most mass spectrometers today utilize some type of electron multiplier as the ion detector. These high-gain, low-noise devices can increase the sensitivity of the mass spectrometer by up to a factor of 100,000. There are four main types of electron multipliers:
- Single channel electron multipliers (Channeltrons, BURLE)
- Spiraltron (BURLE) channel electron multipliers
- Microchannel plate-based detectors
- Discrete dynode multipliers
Conventional electron multipliers originally were designed to operate in vacuums of 1016 Torr or better.
Field-portable mass spectrometers will require ion detectors capable of operating reliably under the poor vacuum conditions that will be prevalent in these instruments. The ideal ion detector for portable mass spectrometers will have the following characteristics:
- Be small and light weight
- Operate at millitorr pressures or higher
- Produce high gain and maintain low noisewhile operating at elevated pressures
- Exhibit long detector lifetime
- Withstand periodic venting to atmosphere
- Tolerate storage at atmospheric pressure
Electron Multipliers Operation in Poor Vacuum Conditions
When electron multipliers are operated in poor vacuum they can become noisy due to ion feedback. Ion feedback results when secondary electrons collide with residual gas molecules,resulting in the generation of positive ions. Positive ions are accelerated to the negative electrode of the multiplier. Upon collision with a dynode (discrete dynode multiplier) or channel wall (Magnums, single-channel electron multipliers, and microchannel plates), additional secondary electrons will be produced. This process results in the multiplier producing an output signal in the absence of any true ion signal. If the pressure is high enough, ions can be created by the strong electric field within the multiplier. The maximum operating pressure of an electron multiplier can be determined, therefore, by observing multiplier background noise under increasing pressure conditions. The operation of electron multipliers in the poor vacuum conditions that will be prevalent in portable instruments has not been widely studied.
Several forms of electron multipliers have been characterized under abnormally high operating pressure conditions. Each form of electron multiplier was loaded into a specially designed vacuum chamber that was capable of modulating the chamber pressure. The multipliers were tuned to produce a gain of 1 million. (The output signal was 1 million times greater than the input ion signal.) Multiplier performance then was characterized at various operating pressures. Figure 1 summarizes how the noise of each type of multiplier varies with pressure.

Figure 1: Plot of electron multiplier noise as a function of vacuum pressure.
The results indicate that the discrete dynode multiplier was the least tolerant of elevating the chamber pressure becoming noisy when the pressure entered the mid 10–5 Torr range.
The most tolerant multiplier was the 2-μm pore microchannel plate followed by the Spiraltron, which operated at high gain and low noise well into the 10–2 Torr range.
Operational lifetime is another critical performance parameter for electron multipliers operated at elevated vacuum pressures. Typical electron multiplier lifetime for benchtop instruments is about one year. Operational lifetime testing also has been investigated (2). Figure 2 illustrates some typical results for a microchannel plate ion detector operated at pressures above 10–3 Torr.
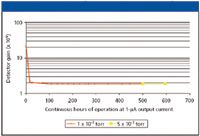
Figure 2: Operational lifetime test results for a 5-μm pore microchannel plate ion detector operated at pressures above 10â3 Torr.
The results indicate that after the characteristic conditioning of the surface at the beginning of the test, the multiplier operation remained stable for hundreds of hours of operation. Other background gases have indicated similar performance (2).
As our world continues to change, and more applications are identified, miniaturization of mass spectrometers will continue. The room-sized instruments of yesterday have been replaced by instruments the size of shoe boxes, while endusers dream of mass spectrometers the size of cell phones. Helping to maintain the sensitivity of these miniature instruments is a new generation of high-pressure tolerant electron multipliers.
Bruce N. Laprade is director of engineering and quality assurance with BURLE Electro-Optics, Inc., Sturbridge, Massachusetts. He can be contacted at: lapradeb@burle-eo.com
References
(1) Hoffmann, Charette, and Stroobant, Mass Spectrometry Principles and Applications (John Wiley and Sons, Hoboken, New Jersey), p. 7.
(2) B. Laprade, Cochran, R. Prunier, and Leffingwell, "Progress on the Development of an Atmospheric Pressure Ion Detector," Paper 200-25P, Pittcon 2006, Orlando, Florida.

AI Shakes Up Spectroscopy as New Tools Reveal the Secret Life of Molecules
April 14th 2025A leading-edge review led by researchers at Oak Ridge National Laboratory and MIT explores how artificial intelligence is revolutionizing the study of molecular vibrations and phonon dynamics. From infrared and Raman spectroscopy to neutron and X-ray scattering, AI is transforming how scientists interpret vibrational spectra and predict material behaviors.
Real-Time Battery Health Tracking Using Fiber-Optic Sensors
April 9th 2025A new study by researchers from Palo Alto Research Center (PARC, a Xerox Company) and LG Chem Power presents a novel method for real-time battery monitoring using embedded fiber-optic sensors. This approach enhances state-of-charge (SOC) and state-of-health (SOH) estimations, potentially improving the efficiency and lifespan of lithium-ion batteries in electric vehicles (xEVs).
New Study Provides Insights into Chiral Smectic Phases
March 31st 2025Researchers from the Institute of Nuclear Physics Polish Academy of Sciences have unveiled new insights into the molecular arrangement of the 7HH6 compound’s smectic phases using X-ray diffraction (XRD) and infrared (IR) spectroscopy.