Advances in the Separation and Detection of As, Cr, and Se Species in Potable Waters Using HPLC Coupled with Dynamic Reaction Cell ICP-MS
Special Issues
Here, the authors discuss a multielement method for the simultaneous determination of inorganic As, Cr, and Se species in potable waters using a HPLC system coupled to a dynamic reaction cell indusctively coupled plasma mass spectrometer.

Since its commercialization almost 25 years ago, inductively coupled plasma-mass spectrometry (ICP-MS) has become the dominant technique for the rapid, multi-element analysis of trace elements in environmental samples (1). In the past 10–15 years, this enormous potential has generated a growing interest in using ICP-MS to determine not just the total metal content of the sample, but also to gain a better understanding of the form or species in which the elements exist (2). Traditionally, the measurement of trace levels of elemental species has been accomplished by separating the species via chromatography and detecting them, one element at a time, as they are eluted. This approach is practical if only one element is being analyzed. However, if multiple elements must be speciated, this scheme is slow and time consuming.
The limitation to multi-element speciation is the separation of the species, not the detection of the elements. Chromatography works on the principle of equilibration between the species of interest, the mobile phase, and the column. Because the chemistries of elements and their species differ, it is difficult to find common conditions capable of separating species of more than one element simultaneously. For example, toxic elements of environmental interest, such as arsenic (As), chromium (Cr), and selenium (Se), have different reaction chemistries, thus requiring different chromatographic conditions to separate.
These elements are also difficult to measure at low levels with ICP-MS because they suffer from polyatomic interferences. However, the development of reaction-cell ICP-MS instruments has allowed As, Cr, and Se to be determined at low levels with relative ease, due to the ability to eliminate the effects of interferences.
Let's take a closer look at the principles of chromatography and reaction cell ICP-MS, with its focus being on the simultaneous speciation analysis of As, Cr, and Se.
Defining the Problem
As mentioned previously, speciation analysis usually is accomplished by analyzing one element at time. As a result, separate chromatographic conditions and injections are required for each element of interest. The key to multi-element speciation is to find chromatographic conditions capable of separating multiple species on the full suite of elements of interest in a single injection.
The analysis chosen for this investigation was the determination of different inorganic species of arsenic, chromium, and selenium in potable waters. This application is important because some of the species are toxic, while others are relatively harmless. For example, the naturally occurring inorganic forms of arsenic (As[III] and As[V]) are highly toxic, while products of biological metabolism, such as arsenobetaine and mono- and dimethylarsenate are relatively innocuous (3). For chromium, naturally occurring trivalent chromium (Cr[III]) is an essential nutrient necessary to sustain life, whereas hexavalent chromium (Cr[VI]) resulting from anthropogenic activity is toxic and highly soluble, making it mobile in the environment (4). Finally, selenium in the form of seleno-proteins is an essential element for human nutrition, whereas many inorganic selenium species (Se[IV], Se[VI], and SeCN– ) have been shown to be poisonous to wildlife (5).
Therefore, the goal of this study is to develop a rapid high performance liquid chromatography (HPLC)–ICP-MS method for the simultaneous separation and measurement of the inorganic species of As, Cr, and Se mentioned earlier. Achieving this goal necessitates finding HPLC conditions capable of separating the species of these three elements in a single injection and determining ICP-MS conditions that can measure trace levels of all species as they are eluted from the HPLC column. This latter goal is accomplished using dynamic reaction cell ICP-MS with a single set of cell conditions capable of eliminating the effects of interferences on As, Cr, and Se. It should be emphasized that no attempt was made to preserve the different species. Although critically important in real world sampling and subsequent analysis, the goal of this exercise was solely to determine chromatographic conditions capable of separating all the species and obtain quantitative results.
Let's first examine the chromatography, focusing on two common forms of separation (ion-exchange and reversed-phase [RP] ion-pairing chromatography) together with two commonly employed HPLC elution techniques (isocratic and gradient). Each process will be defined, and the advantages and disadvantages of each will be discussed in the context of choosing a separation scheme to achieve our analytical goals.
Ion-Exchange Chromatography
In ion-exchange chromatography (IEC), separation is based upon the exchange of ions (anions or cations) between the mobile phase and the ionic sites on a stationary phase bound to a support material in the column. A charged species is bound covalently to the surface of the stationary phase. The mobile phase, typically a buffer, contains a large number of ions that have the opposite charge to the surface-bound ions. These mobile phase ions are referred to as counterions and establish an equilibrium with the stationary phase. Sample ions passing through the column, having the same ionic charge as the counterion, compete with the mobile phase ions for sites on the column, resulting in a disruption of the equilibrium and retention of that analyte. It is the differential competition of various analytes with the mobile phase ions that ultimately produces species separation and chromatographic peaks, as detected by the ICP-MS system. Therefore, analyte retention is based upon the affinity of different ions for the support material and on other solution parameters, including counterion type, ionic strength (buffer concentration), and pH. Varying these mobile phase parameters changes the separation.
The principle of IEC is represented schematically in Figure 1 for an anion exchange scheme. In this figure, the anions are denoted by A– and represent the analyte species, while the counterions are symbolized by X–. The main benefit of this approach is that it has a high tolerance to matrix components, while the main disadvantages are that these columns tend to be expensive and are somewhat fragile.
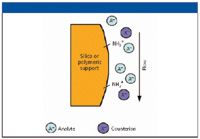
Figure 1: The principle of separation using anion-exchange chromatography.
Reversed-Phase Ion-Pair Chromatography
Ion-pair chromatography (IPC) typically uses an RP column in conjunction with a special type of chemical in the mobile phase, called an ion-pairing reagent. "Reversed-phase" essentially means that the column's stationary phase is nonpolar (less polar, more organic) than the mobile phase solvents. For IPC, the stationary phase is a carbon chain, most often consisting of 8 or 18 carbon atoms (C8 or C18), bonded to a silica support. The mobile phase solvents usually consist of water mixed with a water-miscible organic solvent, such as acetonitrile or methanol.
The ion-pairing reagent is a compound that has both an organic and an ionic end. To promote ionic interaction, the ionic end should have the opposite charge as that of the analytes of interest. For anionic IPC, a commonly used ion-pairing reagent is tetrabutylammonium hydroxide (TBAOH) and, for cationic IPC, hexanesulfonic acid (HSA) often is chosen. In principle, the ionized–ionizable analytes interact with the ionic end of the ion-pairing reagent, while the ion-pairing reagent's organic end interacts with the C18 stationary phase. Retention and separation selectivity are primarily affected by characteristics of the mobile phase, including pH, selection–concentration of ion-pairing reagent, ionic strength, as well as the use of additional mobile phase modifiers. With this scheme, an RP column acts like an ion-exchange column.
The benefits of this type of separation are that the columns used are generally less expensive and tend to be more rugged than ion-exchange columns. The main disadvantages, compared with IEC, are that the separation may not be as good and there may be less tolerance to high sample matrix concentrations. A simplified representation of this approach is shown in Figure 2.
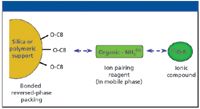
Figure 2: Principle of ion-pair chromatography (anionic mode).
Column Material
As evidenced in the previous discussions, several variables of the mobile phase can be modified to affect separations, both by IEC and RP IPC. A very important consideration when choosing a column is the pH of the mobile phase used to attain the separation. The pH is also critical in the selection of the column for both IEC and RP IPC because the support materials can be pH sensitive. One of the most common column materials is silica because it is inexpensive, rugged, and has been used for a long time. The main disadvantage of silica columns is that they are only useful over a pH range of 2–8; outside this range, the silica dissolves. For applications requiring pHs outside of this range, polymer columns are commonly used. Polymer columns are useful over the entire pH range. The downside to these columns is that they are typically more expensive and fragile. Columns consisting of other support materials also are available.
Isocratic or Gradient Elution
Another consideration is how best to elute the species from the column. This can be accomplished using either an isocratic or gradient elution scheme. Isocratic refers to using a constant solvent composition throughout the analysis: the solvent composition never changes. Because of this, samples can be injected immediately after the preceding one has been eluted. This type of elution can be performed on all HPLC pumping systems. The advantages of isocratic elutions are simplicity and higher sample throughput.
Gradient elutions involve varying the mobile phase composition over time, usually changing the organic content, pH, concentration of the buffer, or changing the buffer all together. Usually this is accomplished by having two (or more) bottles of mobile phases connected to the HPLC pump. The pump is then programmed to vary the amount of each mobile phase component. The mobile phase components are mixed on-line before reaching the column. As a result, a more complex pump is required to handle this task.
The reason that gradient elutions are used is that the mobile phase composition can be varied to finetune the separation. As a result, component separations are better and chromatograms are generally shorter than with isocratic elutions. However, overall sample throughput is much lower than with isocratic separations because of the variation in mobile phase composition. The reason for this is that after an elution is complete, the mobile phase must be changed back to its original composition as at the start of the chromatogram. This means that the equilibrium between the mobile phase and column must be re-established before the next sample can be injected. If the equilibrium is not established, the peaks will be eluted at different times than the previous sample. The equilibration time varies with column, but is usually between 5 and 30 min.
Because of the low sample throughput with gradient elutions and the complexity of method development, it is often best to try first to develop an isocratic separation method. If the required separations cannot be obtained, then a gradient method should be used. Therefore, based upon work not presented in this study, the simultaneous separation of As, Cr, and Se species of environmental interest was accomplished with ion-pairing chromatography using an isocratic separation scheme.
Optimizing Dynamic Reaction Cell ICP-MS Conditions
The principles of dynamic reaction cell technology have been described many times in the open literature (6–8), but a brief description follows. An enclosed rf-only quadrupole is positioned after the ion optics and pressurized with a reactive gas, such as ammonia (NH3), methane (CH4), or oxygen (O2). By a number of different ion–molecule reaction mechanisms, the gas reacts with either the interfering ions to form products that will not interfere with the analyte, or with the analyte to form a polyatomic ion at a different mass, away from the interference. The analyte mass then emerges from the dynamic reaction cell free of its interference and is guided into the analyzer quadrupole for conventional mass separation. These processes are represented schematically in Figure 3. By optimizing the reaction cell quadrupole electrical fields, unwanted reaction by-products, which could potentially lead to new interferences, are prevented. This cleansing process, known as "chemical resolution," means that every time an analyte and interfering ions enter the dynamic reaction cell, the bandpass of the quadrupole is optimized for that specific mass and then changed on-the-fly for the next analyte (6). The benefit of this approach for multi-element analysis is that the instrument can be operated in both dynamic reaction cell and normal modes: different gases and gas flows can be used for elements which have interferences, while noninterfered elements can be analyzed in standard mode (that is, no reaction gas).
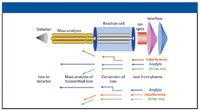
Figure 3: Principles of dynamic reaction cell ICP-MS.
Polyatomic Interferences
As, Cr, and Se are notoriously difficult elements for ICP-MS analysis because their major isotopes suffer from argon-based polyatomic interferences. Arsenic has only one isotope (m/z 75), which is difficult to quantify in chloride-containing samples because of the presence of 40Ar35Cl+. Low-level chromium analysis is difficult due to the presence of 40Ar12C+ at m/z 52, the major Cr isotope. This interference is always present, but is especially strong in samples having an organic content. The argon dimers, (40Ar40Ar+40Ar38Ar+) at masses 80 and 78 interfere with the major isotopes of Se at mass 80 and 78, respectively. The effects of these interferences have been reduced with conventional ICP-MS instruments, but these strategies also hinder determination of the analytes, thus, not offering significant benefits for trace level analyses.
However, by using a suitable reaction gas together with optimization of the quadrupole electrical fields, a dynamic reaction cell can virtually eliminate the effect of these polyatomic interferences. After reviewing ion–molecule reaction mechanisms, optimization studies were carried out using oxygen as the reaction gas. Both 52Cr+ and 80Se+ ions do not react with oxygen, while the polyatomic ions 40Ar12C+ and 40Ar40Ar+ do. The ion–molecule reactions for both elements and their interferences are shown in Table I.

Table I: Ion-molecule reactions for chromium and selenium
In the case of arsenic, advantage is taken of the dynamic reaction cell's ability to control the chemistry within the reaction cell. Oxygen reacts with 75As+ to form 75As16O+ at m/z 91, while the chloride interferences at m/z 75 (40Ar35Cl+, 40Ca35Cl+) do not react with O2. This scheme has been well-documented in the literature and has proven to be a novel way of determining arsenic in a chloride matrix (9,10).

Figure 4: Optimization of the oxygen gas flow in the conversion of 75As to 75As16O+.
Optimization of the oxygen gas flow for the conversion of 75As+ to 75As16O+ is shown in Figure 4. It is clearly evident that as the O2 flow increases, the 75As+ signal decreases, while the 75As16O+ signal increases, up to a flow of 0.45 mL/min, at which the difference in signals is at a maximum.
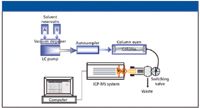
Figure 5: A schematic of the HPLCâICP-MS set-up used in this study.
Setting Up the HPLC–ICP-MS Method
All work was done on a Series 200 HPLC system, consisting of a quaternary LC pump, autosampler, column oven, and vacuum degasser (all PerkinElmer LAS, Shelton, Connecticut) coupled with an ELAN DRC II ICP mass spectrometer (PerkinElmer, SCIEX, Concord, Ontario, Canada). A schematic of the fully integrated computer-controlled system is shown in Figure 5. As mentioned previously, the decision was made to use RP ion-pairing chromatography with isocratic elution to separate the inorganic forms of arsenic (As+3 and As+5), chromium (Cr+3 and Cr+6) and selenium (Se+4, Se+6, and SeCN–). It is important to emphasize that if these species were being measured on a single element basis, the optimum chromatographic and ICP-MS conditions would be different. However, the goal of this study was to determine all the species in a single multielement run, so the method could be applied to a routine, high throughput environment. With this in mind, Table II shows the chromatographic conditions used for the analysis. The 3.3-cm-long column was packed with a C8 hydrocarbon material (3-μm particle size). The mobile phase was a mixture of tetrabutylammonium hydroxide, ammonium acetate, the potassium salt of EDTA and methanol. The pH was adjusted to 7.5 (before the addition of methanol) using 10% nitric acid and 10% ammonium hydroxide. Sample preparation consisted of dilution with the mobile phase and heating at 50–55 °C to speed the formation of the Cr(III)–EDTA complex. For each analysis, 50 μL of sample was injected onto the column.

Table II: Chromatographic conditions used for the simultaneous separation of various inorganic species of As, Cr, and Se
The ICP-MS operating parameters are shown in Table III. Once again, one set of compromise conditions was used so that the analysis could be carried out on a multi-element basis for the transient signal. It should also be noted that although the optimum oxygen gas flow for the determination of arsenic was in the order of 0.4–0.5 mL/min, a higher gas flow was used for the best compromise for reducing the ArC+ and Ar2+ interferences on Cr+ and Se+ respectively, while still sustaining formation of AsO+.
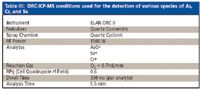
Table III: DRC-ICP-MS conditions used for the detection of various species of As, Cr, and Se
Results
The chromatograms for a 1-μg/L mixed standard containing the species of interest are shown in Figures 6 and 7. These are the same chromatograms, but displayed differently: Figure 6 shows how the signals appear on the screen during an analysis, while Figure 7 shows the chromatograms separately for easier viewing. From Figure 6, the advantage of using a multielement detector is clear: because different masses are being monitored for each element, a complete separation of all seven species does not need to be obtained. For example, the peak for Cr+3 virtually overlaps the As+5 peak, while Cr+6 and Se+6 are eluted at the same time (coeluted). If a single channel detector were being used (as is common in conventional HPLC), these species could not be distinguished. However, because three masses are being monitored, species of different elements, which co-elute, can be distinguished easily.

Figure 6: Chromatograms for all the inorganic species of As, Cr, and Se as they appear on the screen during the elution.
It also should be noted that the higher baseline for the Cr species in Figure 6 (compared with As and Se) is the result of using compromised reaction cell conditions to allow the simultaneous monitoring of all elements. With optimized dynamic reaction cell conditions, the baseline would be much lower, as shown in previous work (11). The elevated baseline does not hamper the measurement of samples containing low levels of chromium, as will be shown later.
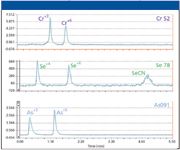
Figure 7: Chromatograms for the inorganic species of As, Cr, and Se shown in Figure 6, but separated.
Figure 8 shows a chromatogram of a bottled water sample purchased in China. The concentrations of the elemental species were determined by measuring peak areas and plotting these against a calibration curve. This sample contains a high level of Se+6, but trace levels of the other species. It should be noted that Cr species are measured at very low levels, which means that the effect of the elevated baseline is negligible.

Figure 8: Chromatogram of a sample of Chinese bottled water, showing the concentrations of all the species present in the sample.
Figure 9 displays chromatograms of a municipal wastewater sample. This sample was filtered (to remove particulates) before undergoing the sample preparation procedure described earlier. The chromatogram on the left shows the presence of trace levels of both Cr species, a high level of As+6, and a peak for selenium that could possibly be Se+6. With HPLC–ICP-MS detection, the peaks are identified by matching retention times of the sample to those in a standard. In this sample, the retention time of the unknown Se peak is close to that of Se+6, but does not match exactly. It is fairly common to see slight retention-time shifts in a sample (as compared with the standard) due to the sample matrix.

Figure 9: Chromatogram of a 2x dilution of (left) a municipal wastewater and (right) the same wastewater spiked with 0.5 mg/L of analyte species.
A common way to determine whether the peak in question is retention time–shifted or another species is to spike the sample with the known species and run it again. The wastewater sample was therefore spiked with 0.5 μg/L of all the elemental species and analyzed; this chromatogram appears on the right side of Figure 9. The spiked chromatogram clearly shows the presence of four selenium peaks, including Se+6. This test proves that the peak found in the sample is in fact another selenium species, which was not determined in this study.
Table IV shows the results of several other water samples which were analyzed by this method, including, bottled water, tap water, well water, and wastewater. It is interesting that many of these samples contain the same unidentified Se species as seen in the wastewater. It should also be noted that speciated water standard reference materials are not available, so the accuracy of the analysis could not be verified by this means.

Table IV: Simultaneous As-Cr-Se speciation analysis of various water samples. All results are in μg/L.
Conclusion
Coupling HPLC with ICP-MS has revolutionized trace metal speciation analysis. By combining the separation capability of HPLC with the selectivity and sensitivity of ICP-MS, many elemental species now can be separated in a single sample injection. When combined with the interference reduction capability of dynamic reaction cell ICP-MS, many of the notoriously difficult elements can be detected at ultratrace levels. Although additional work is needed to verify the methodology, this study has proven that a routine method can be developed to measure trace levels of the inorganic forms of arsenic, chromium, and selenium in potable water samples. By using a fully integrated, computer-controlled HPLC–dynamic reaction cell ICP-MS system and choosing the chromatography and ICP-MS conditions outlined here, a sample can be analyzed for all seven species in approximately 5 min. There is no question that this kind of sample throughput will make the environmental and clinical communities realize that trace element speciation studies now can be carried out in a routine manner.
Kenneth R. Neubauer, Pamela A. Perrone, and Wilhad M. Reuter are with PerkinElmer LAS, Shelton, Connecticut.
Robert Thomas is with Scientific Solutions, Gaithersburg, Maryland.
Please direct correspondence to: kenneth.neubauer@perkinelmer.com
References
(1) D.J. Douglas and R.S. Houk, Prog. Anal. Atomic Spectrosc. 8, 1–18 (1985).
(2) R. Lobinski, I.R. Pereiro, H. Chassaigne, A. Wasik, and J. Szpunar, J. Anal. Atomic Spectrom. 13, 860–867 (1998).
(3) S. Caroli, F. La Torre, F. Petrucci, and N. Violante, Environ. Sci. Pollution Res. 1(4), 205–209 (1994).
(4) A.M. Dattilo and S.G. Miguel, Nutrition Today, July 1, 2003.
(5) K.D. Cafferky, D.R. Richardson, and J. Caruso, Spectroscopy 21(2), 18–24 (2006).
(6) D.R. Bandura, V.I Baranov, and S.D. Tanner, Fresenius J. Anal. Chem. 370, 454–470 (2001).
(7) S.D. Tanner, V.I. Baranov, and S.D. Tanner, Spectrochim. Acta. B 57, 1361–1452 (2002).
(8) S.D. Tanner, V.I. Baranov, and U. Voellkopf, J. Anal. Atomic Spectrom. 15, 1261–1269 (2000).
(9) D.S. Bollinger and A J. Schleisman, Atom. Spectrosc. 20(2), 60–63 (1999).
(10)W. Reuter, L. Davidowski, K. Neubauer, and J. Di Bussolo, PerkinElmer Sciex Application Note D-6736 (2003).
(11)K. Neubauer, W. Reuter, and P. Perrone, PerkinElmer Sciex Application Note 006780 (2003).

High-Speed Laser MS for Precise, Prep-Free Environmental Particle Tracking
April 21st 2025Scientists at Oak Ridge National Laboratory have demonstrated that a fast, laser-based mass spectrometry method—LA-ICP-TOF-MS—can accurately detect and identify airborne environmental particles, including toxic metal particles like ruthenium, without the need for complex sample preparation. The work offers a breakthrough in rapid, high-resolution analysis of environmental pollutants.
Trending on Spectroscopy: The Top Content of 2024
December 30th 2024In 2024, we launched multiple content series, covered major conferences, presented two awards, and continued our monthly Analytically Speaking episodes. Below, you'll find a selection of the most popular content from Spectroscopy over the past year.