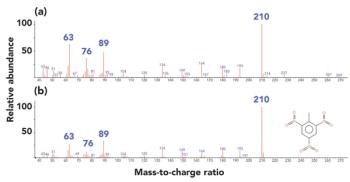
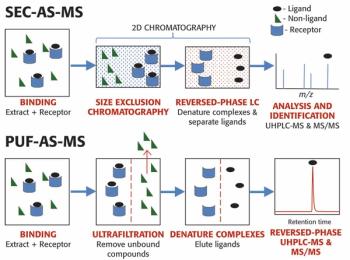
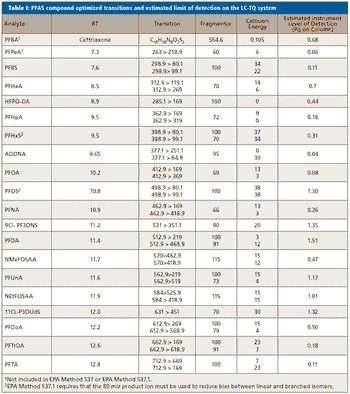
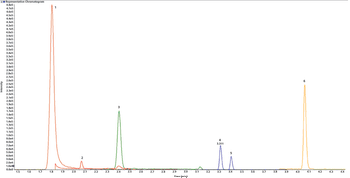
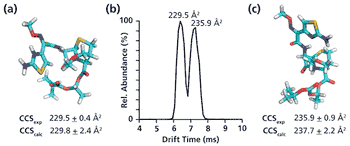
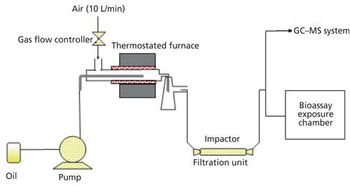
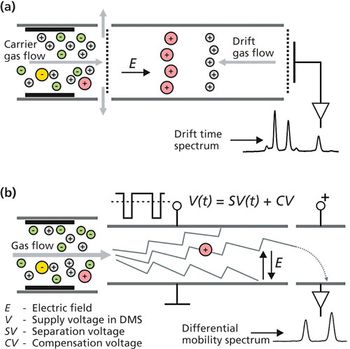
A simple method for extraction and concentration of trace organic compounds found in water for gas chromatography-mass spectrometry (GC-MS) analysis was developed. The method used 25 and 45 mL glass vials with a 5-10 µm thick polymer coatings for extraction of analytes from 20 and 40 mL water samples, respectively. Analytes were subsequently transferred from the polymer coating into an organic solvent, which was reduced in volume to 200-400 µL for analysis. A 10-20 µL sample from the vial was transferred to a tiny coiled stainless steel wire filament using a micro-syringe, or by dipping the coil into the sample. After air evaporation of the solvent, the coil was inserted into the heated injection port of a portable GC-MS system where the analytes were desorbed. Injection using the coiled wire filament eliminated sample discrimination of high boiling point compounds, and minimized system contamination caused by sample matrix residues. The GC-MS contained a new resistively heated column bundle that allowed elution of low-volatility compounds in less than 4 min. Analyses of organochlorine pesticides, polycyclic aromatic hydrocarbons, polychlorinated biphenyl congeners, pyrethroid insecticides, phthalate esters, and n-alkanes in water and wastewater samples were accomplished for low ppb concentrations in less than 10 min total analysis time.